Rit GTPase regulates a p38 MAPK-dependent neuronal survival pathway
- PMID: 23123784
- PMCID: PMC3513593
- DOI: 10.1016/j.neulet.2012.10.036
Rit GTPase regulates a p38 MAPK-dependent neuronal survival pathway
Abstract
Rit, along with Rin and Drosophila Ric, comprises the Rit subfamily of Ras-related small GTPases. Although the cellular functions of many Ras family GTPases are well established, the physiological significance of Rit remains poorly understood. Loss of Rit sensitizes multiple mammalian cell lines and mouse embryonic fibroblasts (MEFs) derived from Rit(-/-) mice to oxidative stress-mediated apoptosis. However, whether Rit-mediated pro-survival signaling extends to other cell types, particularly neurons, is presently unknown. Here, to examine these issues we generated a transgenic mouse overexpressing constitutively active Rit (Rit(Q79L)) exclusively in neurons, under control of the Synapsin I promoter. Active Rit-expressing hippocampal neurons display a dramatic increase in oxidative stress resistance. Moreover, pharmacological inhibitor studies demonstrate that p38 MAPK, rather than a MEK/ERK signaling cascade, is required for Rit-mediated protection. Together, the present studies identify a critical role for the Rit-p38 MAPK signaling cascade in promoting hippocampal neuron survival following oxidative stress.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Figures
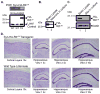

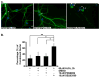
References
-
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

