An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background
- PMID: 23123854
- PMCID: PMC3521494
- DOI: 10.1038/nature11624
An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background
Abstract
People with pale skin, red hair, freckles and an inability to tan--the 'red hair/fair skin' phenotype--are at highest risk of developing melanoma, compared to all other pigmentation types. Genetically, this phenotype is frequently the product of inactivating polymorphisms in the melanocortin 1 receptor (MC1R) gene. MC1R encodes a cyclic AMP-stimulating G-protein-coupled receptor that controls pigment production. Minimal receptor activity, as in red hair/fair skin polymorphisms, produces the red/yellow pheomelanin pigment, whereas increasing MC1R activity stimulates the production of black/brown eumelanin. Pheomelanin has weak shielding capacity against ultraviolet radiation relative to eumelanin, and has been shown to amplify ultraviolet-A-induced reactive oxygen species. Several observations, however, complicate the assumption that melanoma risk is completely ultraviolet-radiation-dependent. For example, unlike non-melanoma skin cancers, melanoma is not restricted to sun-exposed skin and ultraviolet radiation signature mutations are infrequently oncogenic drivers. Although linkage of melanoma risk to ultraviolet radiation exposure is beyond doubt, ultraviolet-radiation-independent events are likely to have a significant role. Here we introduce a conditional, melanocyte-targeted allele of the most common melanoma oncoprotein, BRAF(V600E), into mice carrying an inactivating mutation in the Mc1r gene (these mice have a phenotype analogous to red hair/fair skin humans). We observed a high incidence of invasive melanomas without providing additional gene aberrations or ultraviolet radiation exposure. To investigate the mechanism of ultraviolet-radiation-independent carcinogenesis, we introduced an albino allele, which ablates all pigment production on the Mc1r(e/e) background. Selective absence of pheomelanin synthesis was protective against melanoma development. In addition, normal Mc1r(e/e) mouse skin was found to have significantly greater oxidative DNA and lipid damage than albino-Mc1r(e/e) mouse skin. These data suggest that the pheomelanin pigment pathway produces ultraviolet-radiation-independent carcinogenic contributions to melanomagenesis by a mechanism of oxidative damage. Although protection from ultraviolet radiation remains important, additional strategies may be required for optimal melanoma prevention.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

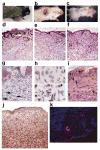
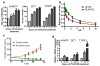
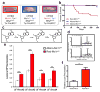
Comment in
-
Cancer: Complexion matters.Nature. 2012 Nov 15;491(7424):342-3. doi: 10.1038/nature11641. Epub 2012 Oct 31. Nature. 2012. PMID: 23123853 No abstract available.
References
-
- Rhodes AR, Weinstock MA, Fitzpatrick TB, Mihm MCJ, Sober AJ. Risk factors for cutaneous melanoma. A practical method of recognizing predisposed individuals. JAMA. 1987;258:3146–3154. - PubMed
-
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–330. - PubMed
-
- Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. MC1R and the response of melanocytes to ultraviolet radiation. Mutation research. 2005;571:133–152. - PubMed
-
- Wenczl E, et al. (Pheo)melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J Invest Dermatol. 1998;111:678–682. - PubMed
-
- Hill HZ, Hill GJ. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000;13 (Suppl 8):140–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

