IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages
- PMID: 23124025
- PMCID: PMC3534796
- DOI: 10.1016/j.freeradbiomed.2012.10.553
IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages
Abstract
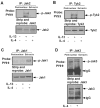
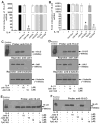
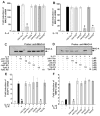
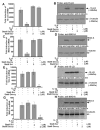
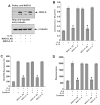
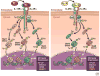
Monocytes/macrophages are innate immune cells that play a crucial role in the resolution of inflammation. In the presence of the Th2 cytokines interleukin-4 (IL-4) and interleukin-13 (IL-13), they display an anti-inflammatory profile and this activation pathway is known as alternative activation. In this study we compare and differentiate pathways mediated by IL-4 and IL-13 activation of human monocytes/macrophages. Here we report differential regulation of IL-4 and IL-13 signaling in monocytes/macrophages starting from IL-4/IL-13 cytokine receptors to Jak/Stat-mediated signaling pathways that ultimately control expression of several inflammatory genes. Our data demonstrate that although the receptor-associated tyrosine kinases Jak2 and Tyk2 are activated after the recruitment of IL-13 to its receptor (containing IL-4Rα and IL-13Rα1), IL-4 stimulates Jak1 activation. We further show that Jak2 is upstream of Stat3 activation and Tyk2 controls Stat1 and Stat6 activation in response to IL-13 stimulation. In contrast, Jak1 regulates Stat3 and Stat6 activation in IL-4-induced monocytes. Our results further reveal that although IL-13 utilizes both IL-4Rα/Jak2/Stat3 and IL-13Rα1/Tyk2/Stat1/Stat6 signaling pathways, IL-4 can use only the IL-4Rα/Jak1/Stat3/Stat6 cascade to regulate the expression of some critical inflammatory genes, including 15-lipoxygenase, monoamine oxidase A (MAO-A), and the scavenger receptor CD36. Moreover, we demonstrate here that IL-13 and IL-4 can uniquely affect the expression of particular genes such as dual-specificity phosphatase 1 and tissue inhibitor of metalloprotease-3 and do so through different Jaks. As evidence of differential regulation of gene function by IL-4 and IL-13, we further report that MAO-A-mediated reactive oxygen species generation is influenced by different Jaks. Collectively, these results have major implications for understanding the mechanism and function of alternatively activated monocytes/macrophages by IL-4 and IL-13 and add novel insights into the pathogenesis and potential treatment of various inflammatory diseases.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





References
-
- Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–2115. - PubMed
-
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. - PubMed
-
- Folcik VA, Aamir R, Cathcart MK. Cytokine modulation of LDL oxidation by activated human monocytes. Arterioscler Thromb Vasc Biol. 1997;17:1954–1961. - PubMed
-
- Allen JB, Wong HL, Costa GL, Bienkowski MJ, Wahl SM. Suppression of monocyte function and differential regulation of IL-1 and IL-1ra by IL-4 contribute to resolution of experimental arthritis. J Immunol. 1993;151:4344–4351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

