Localization and substrate selectivity of sea urchin multidrug (MDR) efflux transporters
- PMID: 23124201
- PMCID: PMC3527970
- DOI: 10.1074/jbc.M112.424879
Localization and substrate selectivity of sea urchin multidrug (MDR) efflux transporters
Abstract
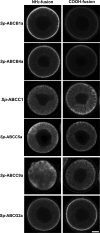
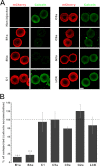
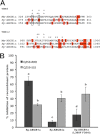
In this study, we cloned, expressed and functionally characterized Stronglycentrotus purpuratus (Sp) ATP-binding cassette (ABC) transporters. This screen identified three multidrug resistance (MDR) transporters with functional homology to the major types of MDR transporters found in humans. When overexpressed in embryos, the apical transporters Sp-ABCB1a, ABCB4a, and ABCG2a can account for as much as 87% of the observed efflux activity, providing a robust assay for their substrate selectivity. Using this assay, we found that sea urchin MDR transporters export canonical MDR susbtrates such as calcein-AM, bodipy-verapamil, bodipy-vinblastine, and mitoxantrone. In addition, we characterized the impact of nonconservative substitutions in the primary sequences of drug binding domains of sea urchin versus murine ABCB1 by mutation of Sp-ABCB1a and treatment of embryos with stereoisomeric cyclic peptide inhibitors (QZ59 compounds). The results indicated that two substitutions in transmembrane helix 6 reverse stereoselectivity of Sp-ABCB1a for QZ59 enantiomers compared with mouse ABCB1a. This suggests that subtle changes in the primary sequence of transporter drug binding domains could fine-tune substrate specificity through evolution.
Figures

References
-
- Dean M., Hamon Y., Chimini G. (2001) The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42, 1007–1017 - PubMed
-
- Leslie E. M., Deeley R. G., Cole S. P. (2005) Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 204, 216–237 - PubMed
-
- Gottesman M. M., Fojo T., Bates S. E. (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

