Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration
- PMID: 23124355
- PMCID: PMC3951144
- DOI: 10.1002/path.4125
Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration
Abstract
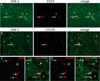
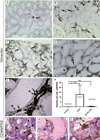
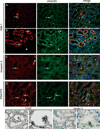
Regeneration of injured tubular cells occurs after acute tubular necrosis primarily from intrinsic renal cells. This may occur from a pre-existing intratubular stem/progenitor cell population or from any surviving proximal tubular cell. In this study, we characterize a CD24-, CD133-, and vimentin-positive subpopulation of cells scattered throughout the proximal tubule in normal human kidney. Compared to adjacent 'normal' proximal tubular cells, these CD24-positive cells contained less cytoplasm, fewer mitochondria, and no brush border. In addition, 49 marker proteins are described that are expressed within the proximal tubules in a similar scattered pattern. For eight of these markers, we confirmed co-localization with CD24. In human biopsies of patients with acute tubular necrosis (ATN), the number of CD24-positive tubular cells was increased. In both normal human kidneys and the ATN biopsies, around 85% of proliferating cells were CD24-positive - indicating that this cell population participates in tubular regeneration. In healthy rat kidneys, the novel cell subpopulation was absent. However, upon unilateral ureteral obstruction (UUO), the novel cell population was detected in significant amounts in the injured kidney. In summary, in human renal biopsies, the CD24-positive cells represent tubular cells with a deviant phenotype, characterized by a distinct morphology and marker expression. After acute tubular injury, these cells become more numerous. In healthy rat kidneys, these cells are not detectable, whereas after UUO, they appeared de novo - arguing against the notion that these cells represent a pre-existing progenitor cell population. Our data indicate rather that these cells represent transiently dedifferentiated tubular cells involved in regeneration.
Copyright © 2012 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
No conflicts of interest were declared.
Figures
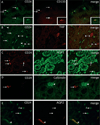
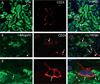
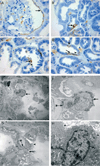

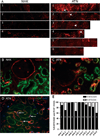
Comment in
-
Of mice and men: the riddle of tubular regeneration.J Pathol. 2013 Apr;229(5):641-4. doi: 10.1002/path.4162. Epub 2013 Feb 11. J Pathol. 2013. PMID: 23299489
References
-
- Vogetseder A, Palan T, Bacic D, et al. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007;292:C807–C813. - PubMed
-
- Vogetseder A, Picard N, Gaspert A, et al. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:C22–C28. - PubMed
-
- Humphreys D, Valerius TM, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

