Somatic rearrangements across cancer reveal classes of samples with distinct patterns of DNA breakage and rearrangement-induced hypermutability
- PMID: 23124520
- PMCID: PMC3561864
- DOI: 10.1101/gr.141382.112
Somatic rearrangements across cancer reveal classes of samples with distinct patterns of DNA breakage and rearrangement-induced hypermutability
Abstract
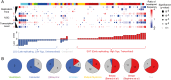
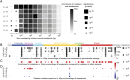
Whole-genome sequencing using massively parallel sequencing technologies enables accurate detection of somatic rearrangements in cancer. Pinpointing large numbers of rearrangement breakpoints to base-pair resolution allows analysis of rearrangement microhomology and genomic location for every sample. Here we analyze 95 tumor genome sequences from breast, head and neck, colorectal, and prostate carcinomas, and from melanoma, multiple myeloma, and chronic lymphocytic leukemia. We discover three genomic factors that are significantly correlated with the distribution of rearrangements: replication time, transcription rate, and GC content. The correlation is complex, and different patterns are observed between tumor types, within tumor types, and even between different types of rearrangements. Mutations in the APC gene correlate with and, hence, potentially contribute to DNA breakage in late-replicating, low %GC, untranscribed regions of the genome. We show that somatic rearrangements display less microhomology than germline rearrangements, and that breakpoint loci are correlated with local hypermutability with a particular enrichment for transversions.
Figures
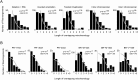

 transversions near breakpoints. Melanoma samples show depletion of
transversions near breakpoints. Melanoma samples show depletion of  transitions near breakpoints due to high
transitions near breakpoints due to high  transitions across the genome.
transitions across the genome.References
-
- Beale RC, Petersen-Mahrt SK, Watt IN, Harris RS, Rada C, Neuberger MS 2004. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: Correlation with mutation spectra in vivo. J Mol Biol 337: 585–596 - PubMed
-
- Benjamini Y, Hochberg Y 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57: 289–300
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
