Association of fibromyalgia with altered skeletal muscle characteristics which may contribute to postexertional fatigue in postmenopausal women
- PMID: 23124535
- PMCID: PMC3558634
- DOI: 10.1002/art.37763
Association of fibromyalgia with altered skeletal muscle characteristics which may contribute to postexertional fatigue in postmenopausal women
Abstract
Objective: To identify muscle physiologic properties that may contribute to postexertional fatigue and malaise in women with fibromyalgia (FM).
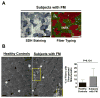
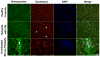
Methods: Healthy postmenopausal women with (n = 11) and without (n = 11) FM, ages 51-70 years, participated in this study. Physical characteristics and responses to self-reported questionnaires were evaluated. Strength loss and tissue oxygenation in response to a fatiguing exercise protocol were used to quantify fatigability and the local muscle hemodynamic profile. Muscle biopsies were performed to assess between-group differences in baseline muscle properties using histochemical, immunohistochemical, and electron microscopic analyses.
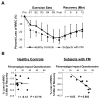
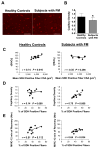
Results: There was no significant difference between healthy controls and FM patients in muscle fatigue in response to exercise. However, self-reported fatigue and pain were correlated with prolonged loss of strength following 12 minutes of recovery in patients with FM. Although there was no difference in percent succinate dehydrogenase (SDH)-positive (type I) and SDH-negative (type II) fibers or in mean fiber cross-sectional area between groups, FM patients exhibited greater variability in fiber size and altered fiber size distribution. In healthy controls only, fatigue resistance was strongly correlated with the size of SDH-positive fibers and hemoglobin oxygenation. In contrast, FM patients with the highest percentage of SDH-positive fibers recovered strength most effectively, and this was correlated with capillary density. However, overall, capillary density was lower in the FM group.
Conclusion: Peripheral mechanisms, i.e., altered muscle fiber size distribution and decreased capillary density, may contribute to postexertional fatigue in FM. Understanding of these defects in fibromyalgic muscle may provide valuable insight with regard to treatment.
Copyright © 2013 by the American College of Rheumatology.
Figures

References
-
- Abeles AM, Pillinger MH, Solitar BM, Abeles M. Narrative review: the pathophysiology of fibromyalgia. Ann Intern Med. 2007;146:726–34. - PubMed
-
- Cho HJ, Skowera A, Cleare A, Wessely S. Chronic fatigue syndrome: an update focusing on phenomenology and pathophysiology. Curr Opin Psychiatry. 2006;19:67–73. - PubMed
-
- Mease P, Arnold LM, Bennett R, Boonen A, Buskila D, Carville S, et al. Fibromyalgia syndrome. J Rheumatol. 2007;34:1415–25. - PubMed
-
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. - PubMed
-
- Yunus MB. Gender differences in fibromyalgia and other related syndromes. J Gend Specif Med. 2002;5:42–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

