Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients
- PMID: 23124782
- PMCID: PMC3562865
- DOI: 10.2215/CJN.08230811
Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients
Abstract
Background and objectives: A dose-finding study was undertaken to investigate the efficacy of PA21, a novel polynuclear iron(III)-oxyhydroxide phosphate binder.
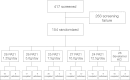
Design, setting, participants, & measurements: In a randomized, active-controlled, multicenter, open-label study at 50 clinical sites in Europe and the United States, hemodialysis patients were randomized to PA21 at dosages of 1.25, 5.0, 7.5, 10.0, or 12.5 g/d or sevelamer-HCl 4.8 g/d for 6 weeks. The primary efficacy endpoint was the change in serum phosphorus concentration from baseline.
Results: There were 154 participants who were randomized and received the study drug. All groups except PA21 1.25 g/d showed a significant decrease in serum phosphorus. Mean decreases in serum phosphorus in PA21 10 g/d and 12.5 g/d were -2.00±1.71 mg/dl and -1.69±1.81 mg/dl, respectively. A similar decrease to sevelamer-HCl (-1.06±1.35 mg/dl) was seen with PA21 5.0 g/d (-1.08±2.12 mg/dl) and 7.5 g/d (-1.25±1.21 mg/d). Overall, 60.9% of participants randomized to PA21 and 57.7% randomized to sevelamer-HCl reported ≥1 adverse event. The most frequent adverse events were hypophosphatemia (18.0%) and discolored feces (11.7%) for the pooled PA21 dose groups, and diarrhea, hypophosphatemia, and hypotension (each 11.5%) for sevelamer-HCl. Discontinuation due to adverse events occurred at a similar rate in PA21-treated (21.1%) and sevelamer-HCl-treated (23.1%) participants.
Conclusions: PA21 5-12.5 g/d significantly reduces serum phosphorus in hemodialysis patients. The 5 g/d and 7.5 g/d dosages showed similar efficacy to 4.8 g/d of sevelamer-HCl. The adverse events rate was similar for PA21 and sevelamer-HCl.
Figures

References
-
- Coladonato JA: Control of hyperphosphatemia among patients with ESRD. J Am Soc Nephrol 16[Suppl 2]: S107–S114, 2005 - PubMed
-
- Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E: A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 33: 694–701, 1999 - PubMed
-
- Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 - PubMed
-
- Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group : Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 - PubMed
-
- Kidney Disease Improving Global Outcomes (KDIGO): Clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Chapter 3.1: Diagnosis of CKD-MBD: Biochemical abnormalities. Kidney Int Supp 76[Suppl 113]: S22–S49, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

