Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism
- PMID: 23125018
- PMCID: PMC3536340
- DOI: 10.1101/cshperspect.a011338
Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism
Abstract
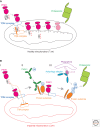
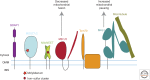
Mutations in Parkin or PINK1 are the most common cause of recessive familial parkinsonism. Recent studies suggest that PINK1 and Parkin form a mitochondria quality control pathway that identifies dysfunctional mitochondria, isolates them from the mitochondrial network, and promotes their degradation by autophagy. In this pathway the mitochondrial kinase PINK1 senses mitochondrial fidelity and recruits Parkin selectively to mitochondria that lose membrane potential. Parkin, an E3 ligase, subsequently ubiquitinates outer mitochondrial membrane proteins, notably the mitofusins and Miro, and induces autophagic elimination of the impaired organelles. Here we review the recent rapid progress in understanding the molecular mechanisms of PINK1- and Parkin-mediated mitophagy and the identification of Parkin substrates suggesting how mitochondrial fission and trafficking are involved. We also discuss how defects in mitophagy may be linked to Parkinson's disease.
Figures
References
-
- Baloh RH, Salavaggione E, Milbrandt J, Pestronk A 2007. Familial parkinsonism and ophthalmoplegia from a mutation in the mitochondrial DNA helicase twinkle. Arch Neurol 64: 998–1000 - PubMed
-
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. 2006. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38: 515–517 - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT 2000. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
