Bacterial quorum sensing: its role in virulence and possibilities for its control
- PMID: 23125205
- PMCID: PMC3543102
- DOI: 10.1101/cshperspect.a012427
Bacterial quorum sensing: its role in virulence and possibilities for its control
Abstract
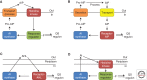
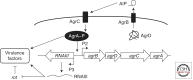
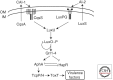
Quorum sensing is a process of cell-cell communication that allows bacteria to share information about cell density and adjust gene expression accordingly. This process enables bacteria to express energetically expensive processes as a collective only when the impact of those processes on the environment or on a host will be maximized. Among the many traits controlled by quorum sensing is the expression of virulence factors by pathogenic bacteria. Here we review the quorum-sensing circuits of Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, and Vibrio cholerae. We outline these canonical quorum-sensing mechanisms and how each uniquely controls virulence factor production. Additionally, we examine recent efforts to inhibit quorum sensing in these pathogens with the goal of designing novel antimicrobial therapeutics.
Figures


References
-
- Agaisse H, Gominet M, Okstad OA, Kolsto AB, Lereclus D 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol Microbiol 32: 1043–1053 - PubMed
-
- Amara N, Mashiach R, Amar D, Krief P, Spieser SA, Bottomley MJ, Aharoni A, Meijler MM 2009. Covalent inhibition of bacterial quorum sensing. J Am Chem Soc 131: 10610–10619 - PubMed
-
- Bassler BL, Wright M, Silverman MR 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: Sequence and function of genes encoding a second sensory pathway. Mol Microbiol 13: 273–286 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
