Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001)
- PMID: 23125443
- PMCID: PMC3532832
- DOI: 10.1093/infdis/jis671
Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001)
Abstract
Background: Adenovirus serotype 26 (Ad26) has been developed as a novel candidate vaccine vector for human immunodeficiency virus type 1 (HIV-1) and other pathogens. The primary safety and immunogenicity data from the Integrated Preclinical/Clinical AIDS Vaccine Development Program (IPCAVD) 001 trial, the first-in-human evaluation of a prototype Ad26 vector-based vaccine expressing clade A HIV-1 Env (Ad26.ENVA.01), are reported concurrently with this article. Here, we characterize in greater detail the humoral and cellular immune responses elicited by Ad26.ENVA.01 in humans.
Methods: Samples from the IPCAVD 001 trial were used for humoral and cellular immunogenicity assays.
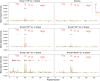
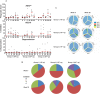
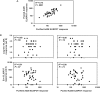
Results: We observed a dose-dependent expansion of the magnitude, breadth, and epitopic diversity of Env-specific binding antibody responses elicited by this vaccine. Antibody-dependent cell-mediated phagocytosis, virus inhibition, and degranulation functional activity were also observed. Env-specific cellular immune responses induced by the vaccine included multiple CD8(+) and CD4(+) T-lymphocyte memory subpopulations and cytokine secretion phenotypes, although cellular immune breadth was limited. Baseline vector-specific T-lymphocyte responses were common but did not impair Env-specific immune responses in this study.
Conclusion: Ad26.ENVA.01 elicited a broad diversity of humoral and cellular immune responses in humans. These data support the further clinical development of Ad26 as a candidate vaccine vector.
Clinical trials registration: NCT00618605.
Figures


References
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. - PubMed
-
- Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U19 AI078526/AI/NIAID NIH HHS/United States
- U01 AI069412/AI/NIAID NIH HHS/United States
- AI066305/AI/NIAID NIH HHS/United States
- AI096040/AI/NIAID NIH HHS/United States
- R01 AI066924/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- AI078526/AI/NIAID NIH HHS/United States
- U19 AI096040/AI/NIAID NIH HHS/United States
- AI069412/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- U19 AI066305/AI/NIAID NIH HHS/United States
- AI060354/AI/NIAID NIH HHS/United States
- AI066924/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

