{2,6-Bis[(2,6-diisopropyl-phosphan-yl)-oxy]-4-fluoro-phenyl-κ(3)P,C(1),P'}(1H-pyrazole-κN(2))nickel(II) hexa-fluoro-phosphate
- PMID: 23125603
- PMCID: PMC3470159
- DOI: 10.1107/S1600536812039207
{2,6-Bis[(2,6-diisopropyl-phosphan-yl)-oxy]-4-fluoro-phenyl-κ(3)P,C(1),P'}(1H-pyrazole-κN(2))nickel(II) hexa-fluoro-phosphate
Abstract
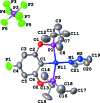
The title compound, [Ni(C(18)H(30)FO(2)P(2))(C(3)H(4)N(2))]PF(6), was prepared by halide abstraction with TlPF(6) in the presence of CH(3)CN in CDCl(3) from the respective neutral pincer chlorido analogue followed by addition of pyrazole. The PO-C-OP pincer ligand acts in typical trans-P(2) tridentate fashion to generate a distorted square-planar nickel structure. The Ni-N(pyrazole) distance is 1.925 (2) Å and the plane of the pyrazole ligand is rotated 56.2 (1)° relative to the approximate square plane surrounding the Ni(II) center in which the pyrazole is bound to the Ni(II) atom through its sp(2)-hybridized N atom. This Ni-N distance is similar to bond lengths in the other reported Ni(II) pincer-ligand square-planar pyrazole complex structures; however, its dihedral angle is significantly larger than any of those for the latter set of pyrazole complexes.
Figures
References
-
- Chen, T., Yang, L., Li, L. & Huang, K.-W. (2012). Tetrahedron, 68, 6152–6157.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Enraf–Nonius (1993). CAD-4-PC Software Enraf–Nonius, Delft, The Netherlands.
-
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
-
- Hoffman, N. W., Stenson, A. C., Sykora, R. E., Traylor, R. K., Wicker, B. F., Riley, S., Dixon, D. A., Marshall, A. G., Kwan, M.-L. & Schroder, P. (2009). Abstracts, Central Regional Meeting, American Chemical Society, Cleveland, OH, United States, May 20–23, CRM-213.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous