The dynamic action of SecA during the initiation of protein translocation
- PMID: 23126322
- PMCID: PMC3685266
- DOI: 10.1042/BJ20121314
The dynamic action of SecA during the initiation of protein translocation
Abstract
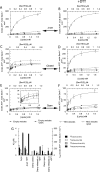
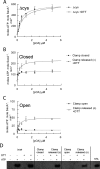
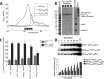
The motor ATPase SecA drives protein secretion through the bacterial Sec complex. The PPXD (pre-protein cross-linking domain) of the enzyme has been observed in different positions, effectively opening and closing a clamp for the polypeptide substrate. We set out to explore the implicated dynamic role of the PPXD in protein translocation by examining the effects of its immobilization, either in the position occupied in SecA alone with the clamp held open or when in complex with SecYEG with the clamp closed. We show that the conformational change from the former to the latter is necessary for high-affinity association with SecYEG and a corresponding activation of ATPase activity, presumably due to the PPXD contacting the NBDs (nucleotide-binding domains). In either state, the immobilization prevents pre-protein transport. However, when the PPXD was attached to an alternative position in the associated SecYEG complex, with the clamp closed, the transport capability was preserved. Therefore large-scale conformational changes of this domain are required for the initiation process, but not for translocation itself. The results allow us to refine a model for protein translocation, in which the mobility of the PPXD facilitates the transfer of pre-protein from SecA to SecYEG.
Figures




References
-
- Brundage L., Hendrick J. P., Schiebel E., Driessen A. J., Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. - PubMed
-
- Breyton C., Haase W., Rapoport T. A., Kuhlbrandt W., Collinson I. Three-dimensional structure of the bacterial protein-translocation complex SecYEG. Nature. 2002;418:662–665. - PubMed
-
- van den Berg B., Clemons W. M., Jr, Collinson I., Modis Y., Hartmann E., Harrison S. C., Rapoport T. A. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

