Large-scale synthesis and self-organization of silver nanoparticles with Tween 80 as a reductant and stabilizer
- PMID: 23127253
- PMCID: PMC3503618
- DOI: 10.1186/1556-276X-7-612
Large-scale synthesis and self-organization of silver nanoparticles with Tween 80 as a reductant and stabilizer
Abstract
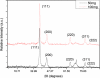
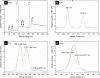
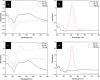
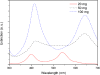
Tween 80 (polysorbate 80) has been used as a reducing agent and protecting agent to prepare stable water-soluble silver nanoparticles on a large scale through a one-pot process, which is simple and environmentally friendly. Silver ions can accelerate the oxidation of Tween 80 and then get reduced in the reaction process. The well-ordered arrays such as ribbon-like silver nanostructures could be obtained by adjusting the reaction conditions. High-resolution transmission electron microscopy confirms that ribbon-like silver nanostructures (approximately 50 nm in length and approximately 2 μm in width) are composed of a large number of silver nanocrystals with a size range of 2 to 3 nm. In addition, negative absorbance around 320 nm in the UV-visible spectra of silver nanoparticles has been observed, probably owing to the instability of nanosized silver colloids.
Figures

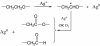





References
-
- Panigrahi S, Basu S, Praharaj S, Pande S, Jana S, Pal A, Ghosh SK, Pal T. Synthesis and size-selective catalysis by supported gold nanoparticles: study on heterogeneous and homogeneous catalytic process. J Phys Chem C. 2007;111:4596–4605.
-
- Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B. 2003;107:668–677.
-
- Taleb A, Petit C, Pileni MP. Optical properties of self-assembled 2D and 3D superlattices of silver nanoparticles. J Phys Chem B. 1998;102:2214–2220. doi: 10.1021/jp972807s. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

