A contemporary concept of the blood-aqueous barrier
- PMID: 23128417
- PMCID: PMC3544162
- DOI: 10.1016/j.preteyeres.2012.10.004
A contemporary concept of the blood-aqueous barrier
Abstract
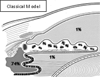
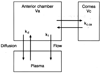
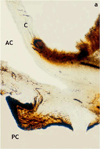
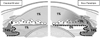
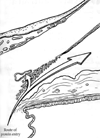
This review traces the evolution of the concept of the blood-aqueous barrier (BAB) during the past 20 years. The Classical model simply stipulated that the tight junctions of the iris vasculature and ciliary epithelium excluded plasma proteins from the aqueous humor (AH). It failed to reconcile the presence of AH protein levels equal to 1% of that found in plasma. Moreover, models of barrier kinetics assumed that the processes of AH secretion and plasma protein entry were directly linked. Thus, elevations of AH protein levels could only be explained by a pathological breakdown of the BAB. Over the last 20 years it has been shown that the plasma proteins in normal AH by-pass the posterior chamber entirely. Instead, these proteins diffuse from the capillaries of ciliary body stroma, into the iris stroma and then into the anterior chamber. This creates a reservoir of plasma-proteins in the iris stroma that is not derived from the iris vessels. This reservoir is prevented from diffusing posteriorly by tight junctions in the posterior iris epithelium. The one-way valve created by the pupil resting on the anterior lens capsule, combined with the continuous, forward flow of AH through the pupil, prevents protein reflux into the posterior chamber. Importantly, in the new paradigm, secretion of AH and the entry of plasma proteins into AH, are semi-independent events. This opens the possibility that AH protein levels could increase in the absence of breakdown of the BAB. Clinical consequences of this new paradigm of the BAB are discussed.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
No conflict of interest exists in the work of the author summarized in this review.
Figures









References
-
- Abraham SV. Miotic iridocyclitis: Its role in the surgical treatment of glaucoma. Am. J. Ophthalmol. 1959;48:634–643. - PubMed
-
- Anjou CI. Physiological variations of the aqueous flare density in normal human eyes. Acta Ophthalmol. (Copenh.) 1961a;39:525–539. - PubMed
-
- Anjou CI. Influence of light on the 24-hour variation in aqueous flare density and intra-ocular pressure in normal rabbits' eyes. Acta Ophthalmol. (Copenh.) 1961b;39:852–873. - PubMed
-
- Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: Challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundamental & Clin. Pharmacol. 2012;26:86–117. - PubMed
-
- Bárány EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest. Ophthalmol. Vis. Sci. 1964;3:135–143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

