Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study
- PMID: 23128859
- PMCID: PMC4667563
- DOI: 10.7326/0003-4819-157-9-201211060-00003
Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study
Abstract
Background: The effects of sulfonylureas and metformin on outcomes of cardiovascular disease (CVD) in type 2 diabetes are not well-characterized.
Objective: To compare the effects of sulfonylureas and metformin on CVD outcomes (acute myocardial infarction and stroke) or death.
Design: Retrospective cohort study.
Setting: National Veterans Health Administration databases linked to Medicare files.
Patients: Veterans who initiated metformin or sulfonylurea therapy for diabetes. Patients with chronic kidney disease or serious medical illness were excluded.
Measurements: Composite outcome of hospitalization for acute myocardial infarction or stroke, or death, adjusted for baseline demographic characteristics; medications; cholesterol, hemoglobin A1c, and serum creatinine levels; blood pressure; body mass index; health care utilization; and comorbid conditions.
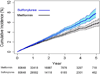
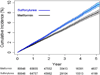
Results: Among 253 690 patients initiating treatment (98 665 with sulfonylurea therapy and 155 025 with metformin therapy), crude rates of the composite outcome were 18.2 per 1000 person-years in sulfonylurea users and 10.4 per 1000 person-years in metformin users (adjusted incidence rate difference, 2.2 [95% CI, 1.4 to 3.0] more CVD events with sulfonylureas per 1000 person-years; adjusted hazard ratio [aHR], 1.21 [CI, 1.13 to 1.30]). Results were consistent for both glyburide (aHR, 1.26 [CI, 1.16 to 1.37]) and glipizide (aHR, 1.15 [CI, 1.06 to 1.26]) in subgroups by CVD history, age, body mass index, and albuminuria; in a propensity score-matched cohort analysis; and in sensitivity analyses.
Limitation: Most of the veterans in the study population were white men; data on women and minority groups were limited but reflective of the Veterans Health Administration population.
Conclusion: Use of sulfonylureas compared with metformin for initial treatment of diabetes was associated with an increased hazard of CVD events or death.
Primary funding source: Agency for Healthcare Research and Quality and the U.S. Department of Health and Human Services.
Figures

Comment in
-
[Cardiovascular events in type 2 diabetes: Sulfonylurea vs. metformin monotherapy].Praxis (Bern 1994). 2013 Feb 13;102(4):237-8. doi: 10.1024/1661-8157/a001193. Praxis (Bern 1994). 2013. PMID: 23399609 German. No abstract available.
Summary for patients in
-
Summaries for patients. How do older diabetes drugs compare in their effects on heart and blood vessel disease?Ann Intern Med. 2012 Nov 6;157(9):I-28. doi: 10.7326/0003-4819-157-9-201211060-00001. Ann Intern Med. 2012. PMID: 23128878 No abstract available.
References
-
- Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. - PubMed
-
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. [Accessed on: March 27, 2012];2011 Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
-
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
