The mitochondrial folylpolyglutamate synthetase gene is required for nitrogen utilization during early seedling development in arabidopsis
- PMID: 23129207
- PMCID: PMC3561033
- DOI: 10.1104/pp.112.203430
The mitochondrial folylpolyglutamate synthetase gene is required for nitrogen utilization during early seedling development in arabidopsis
Abstract
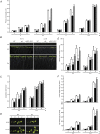
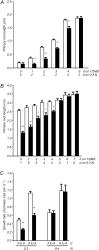
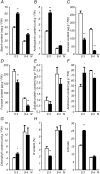
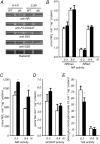
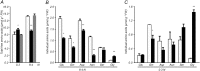
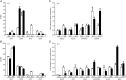
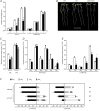
Investigations into the biochemical processes and regulatory mechanisms of nitrogen (N) utilization can aid in understanding how N is used efficiently in plants. This report describes a deficiency in N utilization in an Arabidopsis (Arabidopsis thaliana) transfer DNA insertion mutant of the mitochondrial folylpolyglutamate synthetase gene DFC, which catalyzes the conjugation of glutamate residues to the tetrahydrofolate during folate synthesis. The mutant seedlings displayed several metabolic changes that are typical of plant responses to low-N stress, including increased levels of starch and anthocyanin synthesis as well as decreased levels of soluble protein and free amino acid, as compared with those in wild-type seedlings when external N was sufficient. More striking changes were observed when dfc seedlings were grown under N-limited conditions, including shorter primary roots, fewer lateral roots, higher levels of glycine and carbon-N ratios, and lower N content than those in wild-type seedlings. Gene expression studies in mutant seedlings revealed altered transcript levels of several genes involved in folate biosynthesis and N metabolism. The biochemical and metabolic changes also suggested that N assimilation is drastically perturbed due to a loss of DFC function. The observation that elevated CO(2) partly rescued the dfc phenotypes suggests that the alterations in N metabolism in dfc may be mainly due to a defect in photorespiration. These results indicate that DFC is required for N utilization in Arabidopsis and provide new insight into a potential interaction between folate and N metabolism.
Figures


Similar articles
-
Arabidopsis plastidial folylpolyglutamate synthetase is required for seed reserve accumulation and seedling establishment in darkness.PLoS One. 2014 Jul 7;9(7):e101905. doi: 10.1371/journal.pone.0101905. eCollection 2014. PLoS One. 2014. PMID: 25000295 Free PMC article.
-
Plastidial Folate Prevents Starch Biosynthesis Triggered by Sugar Influx into Non-Photosynthetic Plastids of Arabidopsis.Plant Cell Physiol. 2017 Aug 1;58(8):1328-1338. doi: 10.1093/pcp/pcx076. Plant Cell Physiol. 2017. PMID: 28586467 Free PMC article.
-
The folylpolyglutamate synthetase plastidial isoform is required for postembryonic root development in Arabidopsis.Plant Physiol. 2011 Mar;155(3):1237-51. doi: 10.1104/pp.110.168278. Epub 2011 Jan 13. Plant Physiol. 2011. PMID: 21233333 Free PMC article.
-
Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen.Plant Physiol. 2004 Sep;136(1):2483-99. doi: 10.1104/pp.104.047019. Plant Physiol. 2004. PMID: 15375205 Free PMC article. Review.
-
Planting resilience: One-Carbon metabolism and stress responses.Plant Physiol Biochem. 2025 Jul;224:109966. doi: 10.1016/j.plaphy.2025.109966. Epub 2025 Apr 28. Plant Physiol Biochem. 2025. PMID: 40319586 Review.
Cited by
-
Genetic and physiological regulation of folate in pak choi (Brassica rapa subsp. Chinensis) germplasm.J Exp Bot. 2020 Aug 6;71(16):4914-4929. doi: 10.1093/jxb/eraa218. J Exp Bot. 2020. PMID: 32639001 Free PMC article.
-
The maize brown midrib4 (bm4) gene encodes a functional folylpolyglutamate synthase.Plant J. 2015 Feb;81(3):493-504. doi: 10.1111/tpj.12745. Epub 2015 Jan 9. Plant J. 2015. PMID: 25495051 Free PMC article.
-
Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress.Photosynth Res. 2019 Mar;139(1-3):67-79. doi: 10.1007/s11120-018-0577-x. Epub 2018 Sep 5. Photosynth Res. 2019. PMID: 30187303 Review.
-
Simultaneous extraction and determination of mono-/polyglutamyl folates using high-performance liquid chromatography-tandem mass spectrometry and its applications in starchy crops.Anal Bioanal Chem. 2019 May;411(13):2891-2904. doi: 10.1007/s00216-019-01742-0. Epub 2019 Mar 19. Anal Bioanal Chem. 2019. PMID: 30888468
-
Rice folate enhancement through metabolic engineering has an impact on rice seed metabolism, but does not affect the expression of the endogenous folate biosynthesis genes.Plant Mol Biol. 2013 Nov;83(4-5):329-49. doi: 10.1007/s11103-013-0091-7. Epub 2013 Jun 16. Plant Mol Biol. 2013. PMID: 23771598
References
-
- Andrew KN, Worsfold PJ, Comber M. (1995) On-line flow injection monitoring of ammonia in industrial liquid effluents. Anal Chim Acta 314: 33–43
-
- Blancquaert D, Storozhenko S, Loizeau K, De Steur H, De Brouwer V, Viaene J, Ravanel S, Rébeillé F, Lambert W, Van Der Straeten D. (2010) Folates and folic acid: from fundamental research toward sustainable health. Crit Rev Plant Sci 29: 14–35
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

