Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials
- PMID: 23129208
- PMCID: PMC3655223
- DOI: 10.1007/s10555-012-9398-0
Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials
Abstract
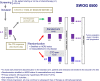
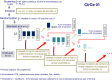
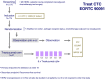
In 2004, circulating tumor cells (CTC) enumeration by the CellSearch® technique at baseline and during treatment was reported to be associated with prognosis in metastatic breast cancer patients. In 2008, the first evidence of the impact of CTC detection by this technique on survival of cM0(i+) patients were reported. These findings were confirmed by other non-interventional studies, whereas CTC were also investigated as a surrogate for tumor biology, mainly for HER2 expression/amplification. The aim of this report is to present the current prospective large interventional studies that have been specifically designed to demonstrate that CTC enumeration/characterization may improve the management of breast cancer patients: STIC CTC METABREAST (France) and Endocrine Therapy Index (USA) assess the CTC-guided hormone therapy vs chemotherapy decision in M1 patients; SWOG0500 (USA) and CirCe01 (France) assess the CTC count changes during treatment in metastatic patients; DETECT III (M1 patients, Germany) and Treat CTC (cM0(i+) patients, European Organization for Research and Treatment of Cancer/Breast International Group) assess the use of anti-HER2 treatments in HER2-negative breast cancer patients selected on the basis of CTC detection/characterization. These trials have different designs in various patient populations but are expected to be the pivotal trials for CTC implementation in the routine management of breast cancer patients.
Figures
References
-
- Liu, M.C., Mego, M., Nakamura, S., Nole, F., Pierga, J., Toi, M., et al. (2011). Clinical validity of circulating tumor cell (CTC) enumeration in 841 subjects with metastatic breast cancer (MBC). Journal of Clinical Oncology, 29, suppl; abstr 10592.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous