Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma
- PMID: 23129742
- PMCID: PMC5569675
- DOI: 10.1200/JCO.2012.43.8820
Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma
Abstract
Purpose: To evaluate induction chemotherapy with docetaxel, cisplatin, and fluorouracil (TPF) followed by surgery and postoperative radiotherapy versus up-front surgery and postoperative radiotherapy in patients with locally advanced resectable oral squamous cell carcinoma (OSCC).
Patients and methods: A prospective open-label phase III trial was conducted. Eligibility criteria included untreated stage III or IVA locally advanced resectable OSCC. Patients received two cycles of TPF induction chemotherapy (docetaxel 75 mg/m(2) on day 1, cisplatin 75 mg/m(2) on day 1, and fluorouracil 750 mg/m(2) on days 1 to 5) followed by radical surgery and postoperative radiotherapy (54 to 66 Gy) versus up-front radical surgery and postoperative radiotherapy. The primary end point was overall survival (OS). Secondary end points included local control and safety.
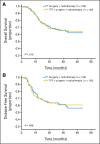
Results: Of the 256 patients enrolled onto this trial, 222 completed the full treatment protocol. There were no unexpected toxicities, and induction chemotherapy did not increase perioperative morbidity. The clinical response rate to induction chemotherapy was 80.6%. After a median follow-up of 30 months, there was no significant difference in OS (hazard ratio [HR], 0.977; 95% CI, 0.634 to 1.507; P = .918) or disease-free survival (HR, 0.974; 95% CI, 0.654 to 1.45; P = .897) between patients treated with and without TPF induction. Patients in the induction chemotherapy arm with a clinical response or favorable pathologic response (≤ 10% viable tumor cells) had superior OS and locoregional and distant control.
Conclusion: Our study failed to demonstrate that TPF induction chemotherapy improves survival compared with up-front surgery in patients with resectable stage III or IVA OSCC.
Trial registration: ClinicalTrials.gov NCT01542931.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures




Comment in
-
Neoadjuvant versus induction chemotherapy: more than semantics.J Clin Oncol. 2013 Aug 10;31(23):2971-2. doi: 10.1200/JCO.2013.50.2674. Epub 2013 Jun 3. J Clin Oncol. 2013. PMID: 23733777 No abstract available.
-
[No improvement in results after surgery, postoperative radiation therapy and neoadjuvant chemotherapy with TPF in locally advanced, resectable squamous cell carcinoma of the oral cavity ].Strahlenther Onkol. 2013 Aug;189(8):702-3. doi: 10.1007/s00066-013-0384-4. Strahlenther Onkol. 2013. PMID: 23771678 German. No abstract available.
References
-
- Kademani D: Oral cancer Mayo Clin Proc 82:878–887,2007 - PubMed
-
- Petersen PE: The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of WHO Global Oral Programme Community Dent Oral Epidemiol 31:3–23,2003. suppl 1 - PubMed
-
- Neville BW, Day TA: Oral cancer and precancerous lesions CA Cancer J Clin 52:195–215,2002 - PubMed
-
- Parkin DM Bray F Ferlay J, etal: Global cancer statistics, 2002 CA Cancer J Clin 55:74–108,2005 - PubMed
-
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers (version 1.2012) http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

