Procontractile G protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling
- PMID: 23129751
- PMCID: PMC3501360
- DOI: 10.1084/jem.20120350
Procontractile G protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling
Abstract
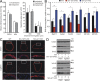
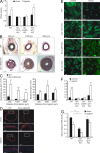
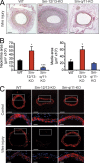
Vascular smooth muscle (Sm) cells (VSMCs) are highly plastic. Their differentiation state can be regulated by serum response factor (SRF), which activates genes involved in Sm differentiation and proliferation by recruiting cofactors, such as members of the myocardin family and ternary complex factors (TCFs), respectively. However, the extracellular cues and upstream signaling mechanisms regulating SRF-dependent VSMC differentiation under in vivo conditions are poorly understood. In this study, we show that the procontractile signaling pathways mediated by the G proteins G(12)/G(13) and G(q)/G(11) antagonistically regulate VSMC plasticity in different models of vascular remodeling. In mice lacking Gα(12)/Gα(13) or their effector, the RhoGEF protein LARG, RhoA-dependent SRF-regulation was blocked and down-regulation of VSMC differentiation marker genes was enhanced. This was accompanied by an excessive vascular remodeling and exacerbation of atherosclerosis. In contrast, Sm-specific Gα(q)/Gα(11) deficiency blocked activation of extracellular signal-regulated kinase 1/2 and the TCF Elk-1, resulting in a reduced VSMC dedifferentiation in response to flow cessation or vascular injury. These data show that the balanced activity of both G protein-mediated pathways in VSMCs is required for an appropriate vessel remodeling response in vascular diseases and suggest new approaches to modulate Sm differentiation in vascular pathologies.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

