Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1-/- mice
- PMID: 23129755
- PMCID: PMC3518636
- DOI: 10.4049/jimmunol.1200585
Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1-/- mice
Abstract
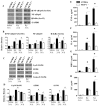
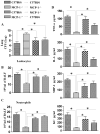
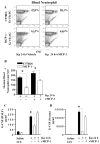
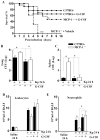
We previously demonstrated that MCP-1 is important for E. coli-induced neutrophil migration to the lungs. However, E. coli neither disseminates nor induces death in mice. Furthermore, the cell types and the host defense mechanisms that contribute to MCP-1-dependent neutrophil trafficking have not been defined. In this study, we sought to explore the cell types and the mechanisms associated with Klebsiella pneumoniae-mediated MCP-1-dependent neutrophil influx. MCP-1(-/-) mice are more susceptible to pulmonary K. pneumoniae infection and show higher bacterial burden in the lungs and dissemination. MCP-1(-/-) mice also display attenuated neutrophil influx, cytokine/chemokine production, and activation of NF-κB and MAPKs following intratracheal K. pneumoniae infection. rMCP-1 treatment in MCP-1(-/-) mice following K. pneumoniae infection rescued impairment in survival, bacterial clearance, and neutrophil accumulation in the lung. Neutrophil numbers in the blood of MCP-1(-/-) mice were associated with G-CSF concentrations in bronchoalveolar lavage fluid and blood. Bone marrow or resident cell-derived MCP-1 contributed to bacterial clearance, neutrophil accumulation, and cytokine/chemokine production in the lungs following infection. Furthermore, exogenous MCP-1 dose dependently increased neutrophil counts and G-CSF concentrations in the blood. Intriguingly, administration of intratracheal rG-CSF to MCP-1(-/-) mice after K. pneumoniae infection rescued survival, bacterial clearance and dissemination, and neutrophil influx in MCP-1(-/-) mice. Collectively, these novel findings unveil an unrecognized role of MCP-1 in neutrophil-mediated host immunity during K. pneumoniae pneumonia and illustrate that G-CSF could be used to rescue impairment in host immunity in individuals with absent or malfunctional MCP-1.
Figures


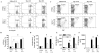


References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. - PubMed
-
- Ho J, Tambyah PA, Paterson DL. Multiresistant Gram-negative infections: a global perspective. Curr Opin Infect Dis. 2010;23:546–553. - PubMed
-
- Baughman RP. Antibiotic resistance in the intensive care unit. Curr Opin Crit Care. 2002;8:430–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

