The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space
- PMID: 23129776
- PMCID: PMC3522305
- DOI: 10.1074/jbc.M112.404467
The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space
Abstract
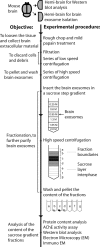
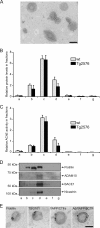
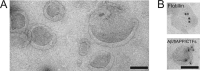
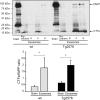
In vitro studies have shown that neuronal cell cultures secrete exosomes containing amyloid-β precursor protein (APP) and the APP-processing products, C-terminal fragments (CTFs) and amyloid-β (Aβ). We investigated the secretion of full-length APP (flAPP) and APP CTFs via the exosome secretory pathway in vivo. To this end, we developed a novel protocol designed to isolate exosomes secreted into mouse brain extracellular space. Exosomes with typical morphology were isolated from freshly removed mouse brains and from frozen mouse and human brain tissues, demonstrating that exosomes can be isolated from post-mortem tissue frozen for long periods of time. flAPP, APP CTFs, and enzymes that cleave both flAPP and APP CTFs were identified in brain exosomes. Although higher levels of both flAPP and APP CTFs were observed in exosomes isolated from the brains of transgenic mice overexpressing human APP (Tg2576) compared with wild-type control mice, there was no difference in the number of secreted brain exosomes. These data indicate that the levels of flAPP and APP CTFs associated with exosomes mirror the cellular levels of flAPP and APP CTFs. Interestingly, exosomes isolated from the brains of both Tg2576 and wild-type mice are enriched with APP CTFs relative to flAPP. Thus, we hypothesize that the exosome secretory pathway plays a pleiotropic role in the brain: exosome secretion is beneficial to the cell, acting as a specific releasing system of neurotoxic APP CTFs and Aβ, but the secretion of exosomes enriched with APP CTFs, neurotoxic proteins that are also a source of secreted Aβ, is harmful to the brain.
Figures

References
-
- Stoorvogel W., Kleijmeer M. J., Geuze H. J., Raposo G. (2002) The biogenesis and functions of exosomes. Traffic 3, 321–330 - PubMed
-
- Février B., Raposo G. (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 16, 415–421 - PubMed
-
- Simpson R. J., Jensen S. S., Lim J. W. (2008) Proteomic profiling of exosomes: current perspectives. Proteomics 8, 4083–4099 - PubMed
-
- Johnstone R. M., Adam M., Hammond J. R., Orr L., Turbide C. (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420 - PubMed
-
- Fauré J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., Sadoul R. (2006) Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

