The role of fructose transporters in diseases linked to excessive fructose intake
- PMID: 23129794
- PMCID: PMC3577529
- DOI: 10.1113/jphysiol.2011.215731
The role of fructose transporters in diseases linked to excessive fructose intake
Abstract
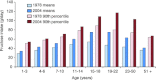
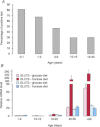
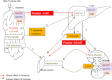
Fructose intake has increased dramatically since humans were hunter-gatherers, probably outpacing the capacity of human evolution to make physiologically healthy adaptations. Epidemiological data indicate that this increasing trend continued until recently. Excessive intakes that chronically increase portal and peripheral blood fructose concentrations to >1 and 0.1 mm, respectively, are now associated with numerous diseases and syndromes. The role of the fructose transporters GLUT5 and GLUT2 in causing, contributing to or exacerbating these diseases is not well known. GLUT5 expression seems extremely low in neonatal intestines, and limited absorptive capacities for fructose may explain the high incidence of malabsorption in infants and cause problems in adults unable to upregulate GLUT5 levels to match fructose concentrations in the diet. GLUT5- and GLUT2-mediated fructose effects on intestinal electrolyte transporters, hepatic uric acid metabolism, as well as renal and cardiomyocyte function, may play a role in fructose-induced hypertension. Likewise, GLUT2 may contribute to the development of non-alcoholic fatty liver disease by facilitating the uptake of fructose. Finally, GLUT5 may play a role in the atypical growth of certain cancers and fat tissues. We also highlight research areas that should yield information needed to better understand the role of these GLUTs in fructose-induced diseases.
Figures
References
-
- Aoyama M, Isshiki K, Kume S, Chin-Kanasaki M, Araki H, Araki S, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Fructose induces tubulointerstitial injury in the kidney of mice. Biochem Biophys Res Commun. 2012;419:244–249. - PubMed
-
- Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–992. - PubMed
-
- Bismut H, Hers HG, Van Schaftingen E. Conversion of fructose to glucose in the rabbit small intestine. A reappraisal of the direct pathway. Eur J Biochem. 1993;213:721–726. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

