A neural model for temporal order judgments and their active recalibration: a common mechanism for space and time?
- PMID: 23130010
- PMCID: PMC3487422
- DOI: 10.3389/fpsyg.2012.00470
A neural model for temporal order judgments and their active recalibration: a common mechanism for space and time?
Abstract
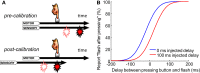
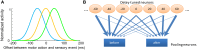
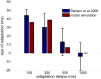
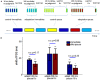
When observers experience a constant delay between their motor actions and sensory feedback, their perception of the temporal order between actions and sensations adapt (Stetson et al., 2006). We present here a novel neural model that can explain temporal order judgments (TOJs) and their recalibration. Our model employs three ubiquitous features of neural systems: (1) information pooling, (2) opponent processing, and (3) synaptic scaling. Specifically, the model proposes that different populations of neurons encode different delays between motor-sensory events, the outputs of these populations feed into rivaling neural populations (encoding "before" and "after"), and the activity difference between these populations determines the perceptual judgment. As a consequence of synaptic scaling of input weights, motor acts which are consistently followed by delayed sensory feedback will cause the network to recalibrate its point of subjective simultaneity. The structure of our model raises the possibility that recalibration of TOJs is a temporal analog to the motion aftereffect (MAE). In other words, identical neural mechanisms may be used to make perceptual determinations about both space and time. Our model captures behavioral recalibration results for different numbers of adapting trials and different adapting delays. In line with predictions of the model, we additionally demonstrate that temporal recalibration can last through time, in analogy to storage of the MAE.
Keywords: motion aftereffect; opponent processing; recalibration; synaptic scaling; temporal order judgment.
Figures


Similar articles
-
Children do not recalibrate motor-sensory temporal order after exposure to delayed sensory feedback.Dev Sci. 2015 Sep;18(5):703-12. doi: 10.1111/desc.12247. Epub 2014 Nov 28. Dev Sci. 2015. PMID: 25444457 Free PMC article.
-
Neural correlates of temporal recalibration to delayed auditory feedback of active and passive movements.Hum Brain Mapp. 2023 Dec 1;44(17):6227-6244. doi: 10.1002/hbm.26508. Epub 2023 Oct 11. Hum Brain Mapp. 2023. PMID: 37818950 Free PMC article.
-
To lead and to lag - forward and backward recalibration of perceived visuo-motor simultaneity.Front Psychol. 2013 Jan 22;3:599. doi: 10.3389/fpsyg.2012.00599. eCollection 2012. Front Psychol. 2013. PMID: 23346063 Free PMC article.
-
Audiotactile interactions in temporal perception.Psychon Bull Rev. 2011 Jun;18(3):429-54. doi: 10.3758/s13423-011-0070-4. Psychon Bull Rev. 2011. PMID: 21400125 Review.
-
On the discrepant results in synchrony judgment and temporal-order judgment tasks: a quantitative model.Psychon Bull Rev. 2012 Oct;19(5):820-46. doi: 10.3758/s13423-012-0278-y. Psychon Bull Rev. 2012. PMID: 22829342 Review.
Cited by
-
Precision-based causal inference modulates audiovisual temporal recalibration.bioRxiv [Preprint]. 2025 Jan 13:2024.03.08.584189. doi: 10.1101/2024.03.08.584189. bioRxiv. 2025. Update in: Elife. 2025 Feb 25;13:RP97765. doi: 10.7554/eLife.97765. PMID: 39553952 Free PMC article. Updated. Preprint.
-
Motor-sensory recalibration modulates perceived simultaneity of cross-modal events at different distances.Front Psychol. 2013 Feb 26;4:46. doi: 10.3389/fpsyg.2013.00046. eCollection 2013. Front Psychol. 2013. PMID: 23549660 Free PMC article.
-
Perceiving the passage of time: neural possibilities.Ann N Y Acad Sci. 2014 Oct;1326(1):60-71. doi: 10.1111/nyas.12545. Epub 2014 Sep 25. Ann N Y Acad Sci. 2014. PMID: 25257798 Free PMC article. Review.
-
Rapid learning and unlearning of predicted sensory delays in self-generated touch.Elife. 2019 Nov 18;8:e42888. doi: 10.7554/eLife.42888. Elife. 2019. PMID: 31738161 Free PMC article.
-
Awareness of Temporal Lag is Necessary for Motor-Visual Temporal Recalibration.Front Integr Neurosci. 2016 Jan 5;9:64. doi: 10.3389/fnint.2015.00064. eCollection 2015. Front Integr Neurosci. 2016. PMID: 26778983 Free PMC article.
References
-
- Albright T. D. (1984). Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 52, 1106–1130 - PubMed
-
- Anstis S. M. (1975). “What does visual perception tell us about visual coding,” in Handbook of Psychobiology, eds. Gazzaniga M., Blakemore C. B. (New York, NY: Academic Press; ), 269–323
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

