Pulmonary blood flow and pulmonary hypertension: Is the pulmonary circulation flowophobic or flowophilic?
- PMID: 23130101
- PMCID: PMC3487301
- DOI: 10.4103/2045-8932.101644
Pulmonary blood flow and pulmonary hypertension: Is the pulmonary circulation flowophobic or flowophilic?
Abstract
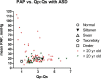
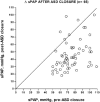
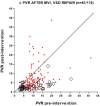
Increased pulmonary blood flow (PBF) is widely thought to provoke pulmonary vascular obstructive disease (PVO), but the impact of wall shear stress in the lung is actually poorly defined. We examined information from patients having cardiac lesions which impact the pulmonary circulation in distinct ways, as well as experimental studies, asking how altered hemodynamics impact the risk of developing PVO. Our results are as follows: (1) with atrial septal defect (ASD; increased PBF but low PAP), shear stress may be increased but there is little tendency to develop PVO; (2) with normal PBF but increased pulmonary vascular resistance (PVR; mitral valve disease) shear stress may also be increased but risk of PVO still low; (3) with high PVR and PBF (e.g., large ventricular septal defect), wall shear stress is markedly increased and the likelihood of developing PVO is much higher than with high PBF or PAP only; and (4) with ASD, experimental and clinical observations suggest that increased PBF plus another stimulus (e.g., endothelial inflammation) may be required for PVO. We conclude that modestly increased wall shear stress (e.g., ASD) infrequently provokes PVO, and likely requires other factors to be harmful. Likewise, increased PAP seldom causes PVO. Markedly increased wall shear stress may greatly increase the likelihood of PVO, but we cannot discriminate its effect from the combined effects of increased PAP and PBF. Finally, the age of onset of increased PAP may critically impact the risk of PVO. Some implications of these observations for future investigations are discussed.
Keywords: pulmonary blood flow; pulmonary hypertension; shear stress.
Conflict of interest statement
Figures
Similar articles
-
Pulmonary vascular resistance as assessed by bicycle stress echocardiography in patients with atrial septal defect type secundum.Circ Cardiovasc Imaging. 2011 May;4(3):237-45. doi: 10.1161/CIRCIMAGING.110.962571. Epub 2011 Feb 25. Circ Cardiovasc Imaging. 2011. PMID: 21357444
-
Elevated Low-Shear Blood Viscosity is Associated with Decreased Pulmonary Blood Flow in Children with Univentricular Heart Defects.Pediatr Cardiol. 2016 Apr;37(4):789-801. doi: 10.1007/s00246-016-1352-4. Epub 2016 Feb 18. Pediatr Cardiol. 2016. PMID: 26888364 Free PMC article.
-
Pulmonary vascular changes induced by congenital obstruction of pulmonary venous return.Ann Thorac Surg. 2000 Jan;69(1):193-7. doi: 10.1016/s0003-4975(99)01079-6. Ann Thorac Surg. 2000. PMID: 10654512
-
Reconciling paradigms of abnormal pulmonary blood flow and quasi-malignant cellular alterations in pulmonary arterial hypertension.Vascul Pharmacol. 2016 Aug;83:17-25. doi: 10.1016/j.vph.2016.01.004. Epub 2016 Jan 22. Vascul Pharmacol. 2016. PMID: 26804008 Review.
-
[Congenital heart disease and acquired valvular lesions in pregnancy].Herz. 2003 May;28(3):227-39. doi: 10.1007/s00059-003-2467-y. Herz. 2003. PMID: 12756480 Review. German.
Cited by
-
Maldevelopment of the Immature Pulmonary Vasculature and Prolonged Patency of the Ductus Arteriosus: Association or Cause?Am J Respir Crit Care Med. 2023 Apr 1;207(7):814-816. doi: 10.1164/rccm.202211-2146ED. Am J Respir Crit Care Med. 2023. PMID: 36470238 Free PMC article. No abstract available.
-
Smooth muscle Cxcl12 activation is associated with vascular remodeling in flow-induced pulmonary hypertension.bioRxiv [Preprint]. 2024 Sep 10:2024.09.10.611870. doi: 10.1101/2024.09.10.611870. bioRxiv. 2024. Update in: J Biol Chem. 2025 Jun;301(6):110207. doi: 10.1016/j.jbc.2025.110207. PMID: 39314465 Free PMC article. Updated. Preprint.
-
Application of a modified clinical classification for pulmonary arterial hypertension associated with congenital heart disease in children: emphasis on atrial septal defects and transposition of the great arteries. An analysis from the TOPP registry.Front Cardiovasc Med. 2024 Feb 2;11:1344014. doi: 10.3389/fcvm.2024.1344014. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38370158 Free PMC article.
-
Pirfenidone ameliorates pulmonary arterial pressure and neointimal remodeling in experimental pulmonary arterial hypertension by suppressing NLRP3 inflammasome activation.Pulm Circ. 2022 Jul 1;12(3):e12101. doi: 10.1002/pul2.12101. eCollection 2022 Jul. Pulm Circ. 2022. PMID: 35833096 Free PMC article.
-
Shear stress unveils patient-specific transcriptional signatures in PAH: Towards personalized molecular diagnostics.Theranostics. 2025 Jan 2;15(5):1589-1605. doi: 10.7150/thno.105729. eCollection 2025. Theranostics. 2025. PMID: 39897541 Free PMC article.
References
-
- Hoffman JI, Rudolph AM, Heymann MA. Pulmonary vascular disease with congenital heart lesions: pathologic features and causes. Circulation. 1981;64:873–7. - PubMed
-
- Chatterjee S, Fisher A. Shear Stress, Cell Signaling, and Pulmonary Vascular Remodeling. In: Yuan JX, Garcia J, Hales C, Rich S, Archer S, West J, editors. Textbook of Pulmonary Vascular Disease. New York: Springer; 2011. p. 796.
LinkOut - more resources
Full Text Sources
Research Materials

