Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models
- PMID: 23130116
- PMCID: PMC3487313
- DOI: 10.1161/JAHA.111.000125
Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models
Abstract
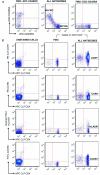
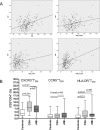
BACKGROUND#ENTITYSTARTX02014;: Adaptive T-cell response is promoted during atherogenesis and results in the differentiation of naïve CD4(+)T cells to effector and/or memory cells of specialized T-cell subsets. Aim of this work was to investigate the relationship between circulating CD4(+)T-cell subsets and atherosclerosis. METHODS AND RESULTS#ENTITYSTARTX02014;: We analyzed 57 subsets of circulating CD4(+)T cells by 10-parameter/8-color polychromatic flow cytometry (markers: CD3/CD4/CD45RO/CD45RA/CCR7/CCR5/CXCR3/HLA-DR) in peripheral blood from 313 subjects derived from 2 independent cohorts. In the first cohort of subjects from a free-living population (n=183), effector memory T cells (T(EM): CD3(+)CD4(+)CD45RA(-)CD45RO(+)CCR7(-) cells) were strongly related with intima-media thickness of the common carotid artery, even after adjustment for age (r=0.27; P<0.001). Of note, a significant correlation between T(EM) and low-density lipoproteins was observed. In the second cohort (n=130), T(EM) levels were significantly increased in patients with chronic stable angina or acute myocardial infarction compared with controls. HLA-DR(+)T(EM) were the T(EM) subpopulation with the strongest association with the atherosclerotic process (r=0.37; P<0.01). Finally, in animal models of atherosclerosis, T(EM) (identified as CD4(+)CD44(+)CD62L(-)) were significantly increased in low-density lipoprotein receptor and apolipoprotein E deficient mice compared with controls and were correlated with the extent of atherosclerotic lesions in the aortic root (r=0.56; P<0.01). CONCLUSIONS#ENTITYSTARTX02014;: Circulating T(EM) cells are associated with increased atherosclerosis and coronary artery disease in humans and in animal models and could represent a key CD4(+)T-cell subset related to the atherosclerotic process. (J Am Heart Assoc. 2012;1:27-41.).
Keywords: C-c chemokine receptor type 7; atherosclerosis; chemokines; coronary artery disease; effector memory T cells.
Figures





Comment in
- J Am Heart Assoc. 1(1):3.
References
-
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. - PubMed
-
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. - PubMed
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. - PubMed
-
- Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res. 2010;86:192–201. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

