Biological Heart Rate Reduction Through Genetic Suppression of Gα(s) Protein in the Sinoatrial Node
- PMID: 23130123
- PMCID: PMC3487376
- DOI: 10.1161/JAHA.111.000372
Biological Heart Rate Reduction Through Genetic Suppression of Gα(s) Protein in the Sinoatrial Node
Abstract
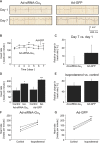
Background: Elevated heart rate represents an independent risk factor for cardiovascular outcome in patients with heart disease. In the sinoatrial node, rate increase is mediated by β(1) adrenoceptor mediated activation of the Gα(s) pathway. We hypothesized that genetic inactivation of the stimulatory Gα(s) protein in the sinoatrial node would provide sinus rate control and would prevent inappropriate heart rate acceleration during β-adrenergic activation.
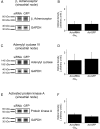
Methods and results: Domestic pigs (n=10) were evenly assigned to receive either Ad-small interfering RNA (siRNA)-Gα(s) gene therapy to inactivate Gα(s) or adenovirus encoding for green fluorescent protein (Ad-GFP) as control. Adenoviruses were applied through virus injection into the sinoatrial node followed by epicardial electroporation, and heart rates were evaluated for 7 days. Genetic inhibition of Gα(s) protein significantly reduced mean heart rates on day 7 by 16.5% compared with control animals (110±8.8 vs 131±9.4 beats per minute; P<0.01). On β-adrenergic stimulation with isoproterenol, we observed a tendency toward diminished rate response in the Ad-siRNA-Gα(s) group (Ad-siRNA-Gα(s), +79.3%; Ad-GFP, +61.7%; n=3 animals per group; P= 0.294). Adverse effects of gene transfer on left ventricular ejection fraction (LVEF) were not detected following treatment (LVEF(Ad-siRNA-Gαs), 66%; LVEF(Ad-GFP), 60%).
Conclusions: In this preclinical proof-of-concept study targeted Ad-siRNA-Gα(s) gene therapy reduced heart rates during normal sinus rhythm compared with Ad-GFP treatment and prevented inappropriate rate increase after β-adrenergic stimulation. Gene therapy may provide an additional therapeutic option for heart rate reduction in cardiac disease. (J Am Heart Assoc. 2012;1:jah3-e000372 doi: 10.1161/JAHA.111.000372).
Keywords: electrophysiology; gene therapy; heart failure; heart rate; sinoatrial node.
Figures



Similar articles
-
Genetic suppression of Gαs protein provides rate control in atrial fibrillation.Basic Res Cardiol. 2012 May;107(3):265. doi: 10.1007/s00395-012-0265-5. Epub 2012 Mar 29. Basic Res Cardiol. 2012. PMID: 22457123
-
Overexpression of wild-type Galpha(i)-2 suppresses beta-adrenergic signaling in cardiac myocytes.FASEB J. 2003 Mar;17(3):523-5. doi: 10.1096/fj.02-0660fje. Epub 2003 Jan 21. FASEB J. 2003. PMID: 12631586
-
Role of force--frequency relation during AV-block, sinus node block and beta-adrenoceptor block in conscious animals.Basic Res Cardiol. 2004 Sep;99(5):360-71. doi: 10.1007/s00395-004-0481-8. Epub 2004 Jul 23. Basic Res Cardiol. 2004. PMID: 15338245
-
Inappropriate sinus tachycardia: a review.Rev Cardiovasc Med. 2021 Dec 22;22(4):1331-1339. doi: 10.31083/j.rcm2204139. Rev Cardiovasc Med. 2021. PMID: 34957774 Review.
-
Impact of physiologic pacing versus right ventricular pacing among patients with left ventricular ejection fraction greater than 35%: A systematic review for the 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society.Heart Rhythm. 2019 Sep;16(9):e280-e298. doi: 10.1016/j.hrthm.2018.10.035. Epub 2018 Nov 6. Heart Rhythm. 2019. PMID: 30412776
Cited by
-
The in vivo regulation of heart rate in the murine sinoatrial node by stimulatory and inhibitory heterotrimeric G proteins.Am J Physiol Regul Integr Comp Physiol. 2013 Aug 15;305(4):R435-42. doi: 10.1152/ajpregu.00037.2013. Epub 2013 May 22. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23697798 Free PMC article.
-
Improving lipoprotein profiles by liver-directed gene transfer of low density lipoprotein receptor gene in hypercholesterolaemia mice.J Genet. 2016 Jun;95(2):311-6. doi: 10.1007/s12041-016-0636-z. J Genet. 2016. PMID: 27350674
-
Cardiac gene therapy with adeno-associated virus-based vectors.Curr Opin Cardiol. 2017 May;32(3):275-282. doi: 10.1097/HCO.0000000000000386. Curr Opin Cardiol. 2017. PMID: 28169951 Free PMC article.
-
Atrial fibrillation complicated by heart failure induces distinct remodeling of calcium cycling proteins.PLoS One. 2015 Mar 16;10(3):e0116395. doi: 10.1371/journal.pone.0116395. eCollection 2015. PLoS One. 2015. PMID: 25775120 Free PMC article.
-
Gene Therapy Approaches to Biological Pacemakers.J Cardiovasc Dev Dis. 2018 Oct 19;5(4):50. doi: 10.3390/jcdd5040050. J Cardiovasc Dev Dis. 2018. PMID: 30347716 Free PMC article. Review.
References
-
- Fox K, Ford I, Steg PG, Tendera M, Ferrari RBEAUTIFUL Investigators Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807-816 - PubMed
-
- Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari RBEAUTIFUL investigators Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817-821 - PubMed
-
- Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Relationship between ivabradine treatment and cardiovascular outcomes in patients with stable coronary artery disease and left ventricular systolic dysfunction with limiting angina: a subgroup analysis of the randomized, controlled BEAUTIFUL trial. Eur Heart J. 2009;30:2337-2345 - PubMed
-
- Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi LSHIFT Investigators Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885 - PubMed
-
- Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi LThe SHIFT Investigators Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886-894 - PubMed
LinkOut - more resources
Full Text Sources

