Biological Heart Rate Reduction Through Genetic Suppression of Gα(s) Protein in the Sinoatrial Node
- PMID: 23130123
- PMCID: PMC3487376
- DOI: 10.1161/JAHA.111.000372
Biological Heart Rate Reduction Through Genetic Suppression of Gα(s) Protein in the Sinoatrial Node
Abstract
Background: Elevated heart rate represents an independent risk factor for cardiovascular outcome in patients with heart disease. In the sinoatrial node, rate increase is mediated by β(1) adrenoceptor mediated activation of the Gα(s) pathway. We hypothesized that genetic inactivation of the stimulatory Gα(s) protein in the sinoatrial node would provide sinus rate control and would prevent inappropriate heart rate acceleration during β-adrenergic activation.
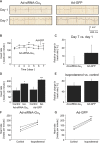
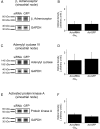
Methods and results: Domestic pigs (n=10) were evenly assigned to receive either Ad-small interfering RNA (siRNA)-Gα(s) gene therapy to inactivate Gα(s) or adenovirus encoding for green fluorescent protein (Ad-GFP) as control. Adenoviruses were applied through virus injection into the sinoatrial node followed by epicardial electroporation, and heart rates were evaluated for 7 days. Genetic inhibition of Gα(s) protein significantly reduced mean heart rates on day 7 by 16.5% compared with control animals (110±8.8 vs 131±9.4 beats per minute; P<0.01). On β-adrenergic stimulation with isoproterenol, we observed a tendency toward diminished rate response in the Ad-siRNA-Gα(s) group (Ad-siRNA-Gα(s), +79.3%; Ad-GFP, +61.7%; n=3 animals per group; P= 0.294). Adverse effects of gene transfer on left ventricular ejection fraction (LVEF) were not detected following treatment (LVEF(Ad-siRNA-Gαs), 66%; LVEF(Ad-GFP), 60%).
Conclusions: In this preclinical proof-of-concept study targeted Ad-siRNA-Gα(s) gene therapy reduced heart rates during normal sinus rhythm compared with Ad-GFP treatment and prevented inappropriate rate increase after β-adrenergic stimulation. Gene therapy may provide an additional therapeutic option for heart rate reduction in cardiac disease. (J Am Heart Assoc. 2012;1:jah3-e000372 doi: 10.1161/JAHA.111.000372).
Keywords: electrophysiology; gene therapy; heart failure; heart rate; sinoatrial node.
Figures



References
-
- Fox K, Ford I, Steg PG, Tendera M, Ferrari RBEAUTIFUL Investigators Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807-816 - PubMed
-
- Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari RBEAUTIFUL investigators Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817-821 - PubMed
-
- Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Relationship between ivabradine treatment and cardiovascular outcomes in patients with stable coronary artery disease and left ventricular systolic dysfunction with limiting angina: a subgroup analysis of the randomized, controlled BEAUTIFUL trial. Eur Heart J. 2009;30:2337-2345 - PubMed
-
- Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi LSHIFT Investigators Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885 - PubMed
-
- Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi LThe SHIFT Investigators Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886-894 - PubMed
LinkOut - more resources
Full Text Sources

