CaMK4 Gene Deletion Induces Hypertension
- PMID: 23130158
- PMCID: PMC3487344
- DOI: 10.1161/JAHA.112.001081
CaMK4 Gene Deletion Induces Hypertension
Abstract
Background: The expression of calcium/calmodulin-dependent kinase IV (CaMKIV) was hitherto thought to be confined to the nervous system. However, a recent genome-wide analysis indicated an association between hypertension and a single-nucleotide polymorphism (rs10491334) of the human CaMKIV gene (CaMK4), which suggests a role for this kinase in the regulation of vascular tone.
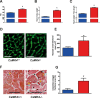
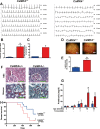
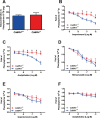
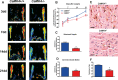
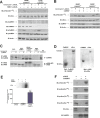
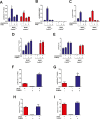
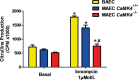
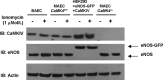
Methods and results: To directly assess the role of CaMKIV in hypertension, we characterized the cardiovascular phenotype of CaMK4(-/-) mice. They displayed a typical hypertensive phenotype, including high blood pressure levels, cardiac hypertrophy, vascular and kidney damage, and reduced tolerance to chronic ischemia and myocardial infarction compared with wild-type littermates. Interestingly, in vitro experiments showed the ability of this kinase to activate endothelial nitric oxide synthase. Eventually, in a population study, we found that the rs10491334 variant associates with a reduction in the expression levels of CaMKIV in lymphocytes from hypertensive patients.
Conclusions: Taken together, our results provide evidence that CaMKIV plays a pivotal role in blood pressure regulation through the control of endothelial nitric oxide synthase activity. (J Am Heart Assoc. 2012;1:e001081 doi: 10.1161/JAHA.112.001081.).
Keywords: angiogenesis; arrhythmia; endothelium; hypertension; hypertrophy.
Figures


References
-
- Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species–activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555-565 - PMC - PubMed
-
- Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476-486 - PubMed
-
- Colomer JM, Mao L, Rockman HA, Means AR. Pressure overload selectively up-regulates Ca2+/calmodulin-dependent protein kinase II in vivo. Mol Endocrinol. 2003;17:183-192 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

