Peroxisome Proliferator-Activated Receptor-α Agonism With Fenofibrate Does Not Suppress Inflammatory Responses to Evoked Endotoxemia
- PMID: 23130172
- PMCID: PMC3487364
- DOI: 10.1161/JAHA.112.002923
Peroxisome Proliferator-Activated Receptor-α Agonism With Fenofibrate Does Not Suppress Inflammatory Responses to Evoked Endotoxemia
Abstract
Background: Data conflict with regard to whether peroxisome proliferator-activated receptor-α agonism suppresses inflammation in humans. We hypothesized that in healthy adults peroxisome proliferator-activated receptor-α agonism with fenofibrate would blunt the induced immune responses to endotoxin (lipopolysaccharide [LPS]), an in vivo model for the study of cardiometabolic inflammation.
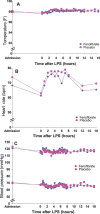
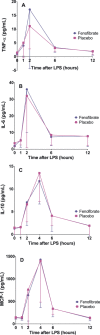
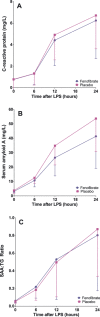
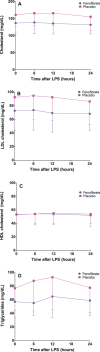
Methods and results: In the Fenofibrate and omega-3 Fatty Acid Modulation of Endotoxemia (FFAME) trial, 36 healthy volunteers (mean age 26±7 years, mean body mass index 24±3 kg/m(2), 44% female, 72% white) were randomized to fenofibrate 145 mg or placebo daily. After 6 to 8 weeks of treatment, subjects underwent a low-dose LPS challenge. Clinical and blood measurements were collected at randomization, before LPS administration, and serially for 24 hours after LPS administration. We examined area under the curve for evoked responses by treatment group. Compared to placebo, but before LPS challenge, fenofibrate reduced total cholesterol and tended to decrease triglycerides, consistent with achieved therapeutic plasma levels of fenofibric acid. In the placebo group, LPS induced a modest inflammatory response with increased cytokines and chemokines (2- to 4-hour post-LPS 8-fold increase in tumor necrosis factor-α, 9-fold increase in interleukin-6, 9-fold increase in interleukin-10, and 10-fold increase in monocyte chemotactic protein-1; all P<0.001) and acute-phase reactants (24-hour post-LPS 15-fold increase in serum amyloid A and 9-fold increase in C-reactive protein; both P<0.001). Compared to placebo, however, fenofibrate did not significantly attenuate LPS-induced levels of plasma cytokines, chemokines, or acute-phase proteins.
Conclusions: These data suggest a lack of systemic antiinflammatory properties of fenofibrate at clinically relevant dosing in humans.
Clinical trial registration: URL: http://clinicaltrials.gov/ct2/show/NCT01048502. Unique identifier: NCT01048502. (J Am Heart Assoc. 2012;1:e002923 doi: 10.1161/JAHA.112.002923.).
Keywords: clinical trials; cytokines; endotoxemia; fenofibrate; inflammation.
Figures

Similar articles
-
Activation of peroxisome proliferator-activated receptor-alpha by fenofibrate prevents myocardial dysfunction during endotoxemia in rats.Crit Care Med. 2007 Mar;35(3):856-63. doi: 10.1097/01.CCM.0000256843.75446.A0. Crit Care Med. 2007. PMID: 17255874
-
Fenofibrate, a peroxisome proliferator-activated receptor α-agonist, blocks lipopolysaccharide-induced inflammatory pathways in mouse liver.Korean J Hepatobiliary Pancreat Surg. 2013 Aug;17(3):89-108. doi: 10.14701/kjhbps.2013.17.3.89. Epub 2013 Aug 31. Korean J Hepatobiliary Pancreat Surg. 2013. PMID: 26155222 Free PMC article.
-
Fenofibrate, a peroxisome proliferator-activated receptor-alpha agonist, blocks steatosis and alters the inflammatory response in a mouse model of inflammation-dioxin interaction.Chem Biol Interact. 2021 Aug 25;345:109521. doi: 10.1016/j.cbi.2021.109521. Epub 2021 May 27. Chem Biol Interact. 2021. PMID: 34052195
-
Suppression of allergen-induced airway inflammation and immune response by the peroxisome proliferator-activated receptor-alpha agonist fenofibrate.Eur J Pharmacol. 2008 Feb 26;581(1-2):177-84. doi: 10.1016/j.ejphar.2007.11.040. Epub 2007 Nov 28. Eur J Pharmacol. 2008. PMID: 18096152
-
Fenofibrate: a review of its lipid-modifying effects in dyslipidemia and its vascular effects in type 2 diabetes mellitus.Am J Cardiovasc Drugs. 2011 Aug 1;11(4):227-47. doi: 10.2165/11207690-000000000-00000. Am J Cardiovasc Drugs. 2011. PMID: 21675801 Review.
Cited by
-
Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence.Int J Mol Sci. 2019 Oct 11;20(20):5055. doi: 10.3390/ijms20205055. Int J Mol Sci. 2019. PMID: 31614690 Free PMC article. Review.
-
Omega-3 PUFA supplementation and the response to evoked endotoxemia in healthy volunteers.Mol Nutr Food Res. 2014 Mar;58(3):601-13. doi: 10.1002/mnfr.201300368. Epub 2013 Nov 5. Mol Nutr Food Res. 2014. PMID: 24190860 Free PMC article. Clinical Trial.
-
Alterations in plasma triglycerides and ceramides: links with cardiac function in humans with type 2 diabetes.J Lipid Res. 2020 Jul;61(7):1065-1074. doi: 10.1194/jlr.RA120000669. Epub 2020 May 11. J Lipid Res. 2020. PMID: 32393551 Free PMC article. Clinical Trial.
-
Human experimental endotoxemia in modeling the pathophysiology, genomics, and therapeutics of innate immunity in complex cardiometabolic diseases.Arterioscler Thromb Vasc Biol. 2015 Mar;35(3):525-34. doi: 10.1161/ATVBAHA.114.304455. Epub 2014 Dec 30. Arterioscler Thromb Vasc Biol. 2015. PMID: 25550206 Free PMC article. Review.
-
Nutrigenomics, the Microbiome, and Gene-Environment Interactions: New Directions in Cardiovascular Disease Research, Prevention, and Treatment: A Scientific Statement From the American Heart Association.Circ Cardiovasc Genet. 2016 Jun;9(3):291-313. doi: 10.1161/HCG.0000000000000030. Epub 2016 Apr 19. Circ Cardiovasc Genet. 2016. PMID: 27095829 Free PMC article. Review.
References
-
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409 - PubMed
-
- Fruchart JC. Peroxisome proliferator–activated receptor-alpha (PPARalpha): at the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205:1-8 - PubMed
-
- Delerive P, De Bosscher K, Besnard S, Berghe WV, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator–activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048-32054 - PubMed
-
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha–leukotriene B4 pathway to inflammation control. Nature. 1996;384:39-43 - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

