Vaginal microbicides for reducing the risk of sexual acquisition of HIV infection in women: systematic review and meta-analysis
- PMID: 23130761
- PMCID: PMC3531270
- DOI: 10.1186/1471-2334-12-289
Vaginal microbicides for reducing the risk of sexual acquisition of HIV infection in women: systematic review and meta-analysis
Abstract
Background: Each year more than two million people are newly infected with HIV worldwide, a majority of them through unprotected vaginal sex. More than half of new infections in adults occur in women. Male condoms and male circumcision reduce the risk of HIV acquisition; but the uptake of these methods is out of the control of women. We therefore aimed to determine the effectiveness of vaginal microbicides (a potential female-controlled method) for prevention of sexual acquisition of HIV in women.
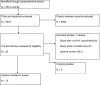
Methods: We conducted a comprehensive search of peer-reviewed and grey literature for publications of randomised controlled trials available by September 2012. We screened search outputs, selected studies, assessed risk of bias, and extracted data in duplicate; resolving differences by discussion and consensus.
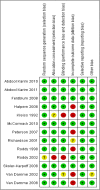
Results: We identified 13 eligible trials that compared vaginal microbicides to placebo. These studies enrolled 35,905 sexually active HIV-negative women between 1996 and 2011; in Benin, Cameroon, Cote d'Ivoire, Ghana, Kenya, Malawi, Nigeria, South Africa, Tanzania, Uganda, Zambia, Zimbabwe, India, Thailand, and the United States of America. A small trial of 889 women found that tenofovir (a nucleotide reverse transcriptase inhibitor) significantly reduces the risk of HIV acquisition (risk ratio [RR] 0.63, 95% confidence intervals [CI] 0.43 to 0.93). Effectiveness data are not yet available from follow-up tenofovir trials being conducted in South Africa, Uganda, and Zimbabwe (1 trial) and multiple sites in South Africa (1 trial). We found no evidence of a significant effect for nonoxynol-9 (5 trials), cellulose sulphate (2 trials), SAVVY (2 trials), Carraguard (1 trial), PRO 2000 (2 trials), and BufferGel (1 trial) microbicides. The pooled RR for the effect of current experimental vaginal microbicides on HIV acquisition in women was 0.97, 95%CI 0.87 to 1.08. Although study results were homogeneous across the different drug classes (heterogeneity P = 0.17, I2 = 27%), the overall intervention effect was not statistically significant. Nonoxynol-9 significantly increased the risk of having adverse genital lesions but no other microbicide led to significant increases in adverse events.
Conclusions: There is not enough evidence at present to recommend vaginal microbicides for HIV prevention. Further high-quality research is needed to confirm the beneficial effects of tenofovir as well as continue the development and testing of new microbicides.
Figures

References
-
- UNAIDS. Global report, UNAIDS report on the global AIDS epidemic 2010. UNAIDS/10.11E / JC1958E. Joint United Nations Programme on HIV/AIDS.
-
- Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BC, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Sag MS, Schechter M, Schooley RT, Thomson MA, Vela S, Yeni PG, Volberding PA. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283:381–390. doi: 10.1001/jama.283.3.381. - DOI - PubMed
-
- Trabattoni D, Lo Caputo S, Biasi M, Seminari E, Di Pierto M, Ravasin G, Mazota F, Maserati R, Clerici M. Modulation of human immunodeficiency virus IHIV)-specific immune response by using efavirenz, nelfinavir, and stavudine in a rescue therapy regimen for HIV-infected, drug-experienced patients. Clin Diagn Lab Immunol. 2000;9:1114–1118. - PMC - PubMed
-
- Wiysonge CS, Shey MS, Kongnyuy EJ, Brocklehurst P. Vaginal microbicide for preventing mother-to-child transmission of HIV infection: no evidence of an effect or evidence of no effect? S Afr Med J. 2007;97:530–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

