Yeast importin-β is required for nuclear import of the Mig2 repressor
- PMID: 23131016
- PMCID: PMC3531251
- DOI: 10.1186/1471-2121-13-31
Yeast importin-β is required for nuclear import of the Mig2 repressor
Abstract
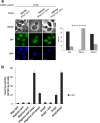
Background: Mig2 has been described as a transcriptional factor that in the absence of Mig1 protein is required for glucose repression of the SUC2 gene. Recently it has been reported that Mig2 has two different subcellular localizations. In high-glucose conditions it is a nuclear modulator of several Mig1-regulated genes, but in low-glucose most of the Mig2 protein accumulates in mitochondria. Thus, the Mig2 protein enters and leaves the nucleus in a glucose regulated manner. However, the mechanism by which Mig2 enters into the nucleus was unknown until now.
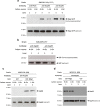
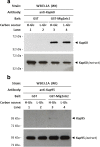
Results: Here, we report that the Mig2 protein is an import substrate of the carrier Kap95 (importin-β). The Mig2 nuclear import mechanism bypasses the requirement for Kap60 (importin-α) as an adaptor protein, since Mig2 directly binds to Kap95 in the presence of Gsp1(GDP). We also show that the Mig2 nuclear import and the binding of Mig2 with Kap95 are not glucose-dependent processes and require a basic NLS motif, located between lysine-32 and arginine-37. Mig2 interaction with Kap95 was assessed in vitro using purified proteins, demonstrating that importin-β, together with the GTP-binding protein Gsp1, is able to mediate efficient Mig2-Kap95 interaction in the absence of the importin-α (Kap60). It was also demonstrated, that the directionality of Mig2 transport is regulated by association with the small GTPase Gsp1 in the GDP- or GTP-bound forms, which promote cargo recognition and release, respectively.
Conclusions: The Mig2 protein accumulates in the nucleus through a Kap95 and NLS-dependent nuclear import pathway, which is independent of importin-α in Saccharomyces cerevisiae.
Figures



Similar articles
-
Nuclear import of the yeast hexokinase 2 protein requires α/β-importin-dependent pathway.J Biol Chem. 2012 Jan 27;287(5):3518-29. doi: 10.1074/jbc.M111.317230. Epub 2011 Dec 7. J Biol Chem. 2012. PMID: 22157003 Free PMC article.
-
Yeast karyopherin Kap95 is required for cell cycle progression at Start.BMC Cell Biol. 2010 Jun 29;11:47. doi: 10.1186/1471-2121-11-47. BMC Cell Biol. 2010. PMID: 20587033 Free PMC article.
-
Nuclear import of dimerized ribosomal protein Rps3 in complex with its chaperone Yar1.Sci Rep. 2016 Nov 7;6:36714. doi: 10.1038/srep36714. Sci Rep. 2016. PMID: 27819319 Free PMC article.
-
Classical nuclear localization signals: definition, function, and interaction with importin alpha.J Biol Chem. 2007 Feb 23;282(8):5101-5. doi: 10.1074/jbc.R600026200. Epub 2006 Dec 14. J Biol Chem. 2007. PMID: 17170104 Free PMC article. Review.
-
Molecular mechanisms of nuclear protein transport.Crit Rev Eukaryot Gene Expr. 1997;7(1-2):61-72. doi: 10.1615/critreveukargeneexpr.v7.i1-2.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9034715 Review.
Cited by
-
Regulation of Aspergillus nidulans CreA-Mediated Catabolite Repression by the F-Box Proteins Fbx23 and Fbx47.mBio. 2018 Jun 19;9(3):e00840-18. doi: 10.1128/mBio.00840-18. mBio. 2018. PMID: 29921666 Free PMC article.
-
The function of importin β1 is conserved in eukaryotes but the substrates may vary in organisms.Plant Signal Behav. 2013 Aug;8(8):e25106. doi: 10.4161/psb.25106. Epub 2013 Jun 3. Plant Signal Behav. 2013. PMID: 23733071 Free PMC article.
-
Hexokinase 2 Is an Intracellular Glucose Sensor of Yeast Cells That Maintains the Structure and Activity of Mig1 Protein Repressor Complex.J Biol Chem. 2016 Apr 1;291(14):7267-85. doi: 10.1074/jbc.M115.711408. Epub 2016 Feb 10. J Biol Chem. 2016. PMID: 26865637 Free PMC article.
-
New twist to nuclear import: When two travel together.Commun Integr Biol. 2013 Jul 1;6(4):e24792. doi: 10.4161/cib.24792. Epub 2013 May 3. Commun Integr Biol. 2013. PMID: 23940825 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

