Fungal infections associated with contaminated methylprednisolone in Tennessee
- PMID: 23131029
- PMCID: PMC4669562
- DOI: 10.1056/NEJMoa1212972
Fungal infections associated with contaminated methylprednisolone in Tennessee
Abstract
Background: We investigated an outbreak of fungal infections of the central nervous system that occurred among patients who received epidural or paraspinal glucocorticoid injections of preservative-free methylprednisolone acetate prepared by a single compounding pharmacy.
Methods: Case patients were defined as patients with fungal meningitis, posterior circulation stroke, spinal osteomyelitis, or epidural abscess that developed after epidural or paraspinal glucocorticoid injections. Clinical and procedure data were abstracted. A cohort analysis was performed.
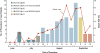
Results: The median age of the 66 case patients was 69 years (range, 23 to 91). The median time from the last epidural glucocorticoid injection to symptom onset was 18 days (range, 0 to 56). Patients presented with meningitis alone (73%), the cauda equina syndrome or focal infection (15%), or posterior circulation stroke with or without meningitis (12%). Symptoms and signs included headache (in 73% of the patients), new or worsening back pain (in 50%), neurologic symptoms (in 48%), nausea (in 39%), and stiff neck (in 29%). The median cerebrospinal fluid white-cell count on the first lumbar puncture among patients who presented with meningitis, with or without stroke or focal infection, was 648 per cubic millimeter (range, 6 to 10,140), with 78% granulocytes (range, 0 to 97); the protein level was 114 mg per deciliter (range, 29 to 440); and the glucose concentration was 44 mg per deciliter (range, 12 to 121) (2.5 mmol per liter [range, 0.7 to 6.7]). A total of 22 patients had laboratory confirmation of Exserohilum rostratum infection (21 patients) or Aspergillus fumigatus infection (1 patient). The risk of infection increased with exposure to lot 06292012@26, older vials, higher doses, multiple procedures, and translaminar approach to epidural glucocorticoid injection. Voriconazole was used to treat 61 patients (92%); 35 patients (53%) were also treated with liposomal amphotericin B. Eight patients (12%) died, seven of whom had stroke.
Conclusions: We describe an outbreak of fungal meningitis after epidural or paraspinal glucocorticoid injection with methylprednisolone from a single compounding pharmacy. Rapid recognition of illness and prompt initiation of therapy are important to prevent complications. (Funded by the Tennessee Department of Health and the Centers for Disease Control and Prevention.).
Figures
References
-
- Carrino JA, Morrison WB, Parker L, Schweitzer ME, Levin DC, Sunshine JH. Spinal injection procedures: volume, provider distribution, and reimbursement in the U.S. Medicare population from 1993 to 1999. Radiology. 2002;225:723–9. - PubMed
-
- Kolbe AB, McKinney AM, Kendi AT, Misselt D. Aspergillus meningitis and discitis from low-back procedures in an immunocompetent patient. Acta Radiol. 2007;48:687–9. - PubMed
-
- Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy — United States, July–November 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1109–12. - PubMed
-
- Hooten WM, Kinney MO, Huntoon MA. Epidural abscess and meningitis after epidural corticosteroid injection. Mayo Clin Proc. 2004;79:682–6. - PubMed
-
- Civen R, Vugia DJ, Alexander R, et al. Outbreak of Serratia marcescens infections following injection of betamethasone compounded at a community pharmacy. Clin Infect Dis. 2006;43:831–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
