Ultrafiltration in decompensated heart failure with cardiorenal syndrome
- PMID: 23131078
- PMCID: PMC3690472
- DOI: 10.1056/NEJMoa1210357
Ultrafiltration in decompensated heart failure with cardiorenal syndrome
Abstract
Background: Ultrafiltration is an alternative strategy to diuretic therapy for the treatment of patients with acute decompensated heart failure. Little is known about the efficacy and safety of ultrafiltration in patients with acute decompensated heart failure complicated by persistent congestion and worsened renal function.
Methods: We randomly assigned a total of 188 patients with acute decompensated heart failure, worsened renal function, and persistent congestion to a strategy of stepped pharmacologic therapy (94 patients) or ultrafiltration (94 patients). The primary end point was the bivariate change from baseline in the serum creatinine level and body weight, as assessed 96 hours after random assignment. Patients were followed for 60 days.
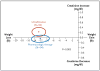
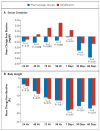
Results: Ultrafiltration was inferior to pharmacologic therapy with respect to the bivariate end point of the change in the serum creatinine level and body weight 96 hours after enrollment (P=0.003), owing primarily to an increase in the creatinine level in the ultrafiltration group. At 96 hours, the mean change in the creatinine level was -0.04±0.53 mg per deciliter (-3.5±46.9 μmol per liter) in the pharmacologic-therapy group, as compared with +0.23±0.70 mg per deciliter (20.3±61.9 μmol per liter) in the ultrafiltration group (P=0.003). There was no significant difference in weight loss 96 hours after enrollment between patients in the pharmacologic-therapy group and those in the ultrafiltration group (a loss of 5.5±5.1 kg [12.1±11.3 lb] and 5.7±3.9 kg [12.6±8.5 lb], respectively; P=0.58). A higher percentage of patients in the ultrafiltration group than in the pharmacologic-therapy group had a serious adverse event (72% vs. 57%, P=0.03).
Conclusions: In a randomized trial involving patients hospitalized for acute decompensated heart failure, worsened renal function, and persistent congestion, the use of a stepped pharmacologic-therapy algorithm was superior to a strategy of ultrafiltration for the preservation of renal function at 96 hours, with a similar amount of weight loss with the two approaches. Ultrafiltration was associated with a higher rate of adverse events. (Funded by the National Heart, Lung, and Blood Institute; ClinicalTrials.gov number, NCT00608491.).
Figures
Comment in
-
Reconsidering ultrafiltration in the acute cardiorenal syndrome.N Engl J Med. 2012 Dec 13;367(24):2351-2. doi: 10.1056/NEJMe1212881. Epub 2012 Nov 6. N Engl J Med. 2012. PMID: 23131079 No abstract available.
-
Heart failure. Drugs outperform ultrafiltration in acute cardiorenal syndrome.Nat Rev Cardiol. 2013 Jan;10(1):2. doi: 10.1038/nrcardio.2012.173. Epub 2012 Nov 20. Nat Rev Cardiol. 2013. PMID: 23165076 No abstract available.
-
Heart failure: Drugs outperform ultrafiltration in acute cardiorenal syndrome.Nat Rev Nephrol. 2013 Jan;9(1):2. doi: 10.1038/nrneph.2012.237. Epub 2012 Nov 20. Nat Rev Nephrol. 2013. PMID: 23165301 No abstract available.
-
Ultrafiltration in heart failure with cardiorenal syndrome.N Engl J Med. 2013 Mar 21;368(12):1159-60. doi: 10.1056/NEJMc1300456. N Engl J Med. 2013. PMID: 23514295 No abstract available.
-
Ultrafiltration in heart failure with cardiorenal syndrome.N Engl J Med. 2013 Mar 21;368(12):1157. doi: 10.1056/NEJMc1300456. N Engl J Med. 2013. PMID: 23514296 No abstract available.
-
Ultrafiltration in heart failure with cardiorenal syndrome.N Engl J Med. 2013 Mar 21;368(12):1157-8. doi: 10.1056/NEJMc1300456. N Engl J Med. 2013. PMID: 23514297 No abstract available.
-
Ultrafiltration in heart failure with cardiorenal syndrome.N Engl J Med. 2013 Mar 21;368(12):1158. doi: 10.1056/NEJMc1300456. N Engl J Med. 2013. PMID: 23514298 No abstract available.
-
Ultrafiltration in heart failure with cardiorenal syndrome.N Engl J Med. 2013 Mar 21;368(12):1158-9. doi: 10.1056/NEJMc1300456. N Engl J Med. 2013. PMID: 23514299 No abstract available.
-
Diuretics or ultrafiltration for acute decompensated heart failure and cardiorenal syndrome?Am J Kidney Dis. 2013 Sep;62(3):453-6. doi: 10.1053/j.ajkd.2013.03.001. Epub 2013 Mar 30. Am J Kidney Dis. 2013. PMID: 23548557 No abstract available.
References
-
- Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–42. - PubMed
-
- Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. - PubMed
-
- Hunt SA, Abraham WT, Chin MH, et al. Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–e479. Erratum, Circulation 2010;121(12)e258. - PubMed
-
- Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–53. - PubMed
-
- Freda BJ, Slawsky M, Mallidi J, Braden GL. Decongestive treatment of acute de-compensated heart failure: cardiorenal implications of ultrafiltration and diuretics. Am J Kidney Dis. 2011;58:1005–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL084890/HL/NHLBI NIH HHS/United States
- U01 HL084891/HL/NHLBI NIH HHS/United States
- U10HL084875/HL/NHLBI NIH HHS/United States
- U01 HL084931/HL/NHLBI NIH HHS/United States
- U01 HL084875/HL/NHLBI NIH HHS/United States
- U10HL084890/HL/NHLBI NIH HHS/United States
- U01 HL084907/HL/NHLBI NIH HHS/United States
- U10HL084861/HL/NHLBI NIH HHS/United States
- U01 HL084899/HL/NHLBI NIH HHS/United States
- U10HL084899/HL/NHLBI NIH HHS/United States
- U10HL084931/HL/NHLBI NIH HHS/United States
- U10HL084891/HL/NHLBI NIH HHS/United States
- U10HL084889/HL/NHLBI NIH HHS/United States
- U10HL084907/HL/NHLBI NIH HHS/United States
- U01 HL084861/HL/NHLBI NIH HHS/United States
- U10 HL084904/HL/NHLBI NIH HHS/United States
- S21 MD000101/MD/NIMHD NIH HHS/United States
- U10HL084877/HL/NHLBI NIH HHS/United States
- U01 HL084877/HL/NHLBI NIH HHS/United States
- U10HL084904/HL/NHLBI NIH HHS/United States
- U01 HL084889/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
