Cellular alterations in human traumatic brain injury: changes in mitochondrial morphology reflect regional levels of injury severity
- PMID: 23131111
- PMCID: PMC3589878
- DOI: 10.1089/neu.2012.2339
Cellular alterations in human traumatic brain injury: changes in mitochondrial morphology reflect regional levels of injury severity
Abstract
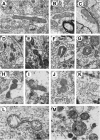
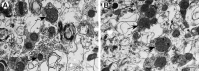
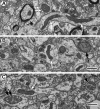
Mitochondrial dysfunction may be central to the pathophysiology of traumatic brain injury (TBI) and often can be recognized cytologically by changes in mitochondrial ultrastructure. This study is the first to broadly characterize and quantify mitochondrial morphologic alterations in surgically resected human TBI tissues from three contiguous cortical injury zones. These zones were designated as injury center (Near), periphery (Far), and Penumbra. Tissues from 22 patients with TBI with varying degrees of damage and time intervals from TBI to surgical tissue collection within the first week post-injury were rapidly fixed in the surgical suite and processed for electron microscopy. A large number of mitochondrial structural patterns were identified and divided into four survival categories: normal, normal reactive, reactive degenerating, and end-stage degenerating profiles. A tissue sample acquired at 38 hours post-injury was selected for detailed mitochondrial quantification, because it best exhibited the wide variation in cellular and mitochondrial changes consistently noted in all the other cases. The distribution of mitochondrial morphologic phenotypes varied significantly between the three injury zones and when compared with control cortical tissue obtained from an epilepsy lobectomy. This study is unique in its comparative quantification of the mitochondrial ultrastructural alterations at progressive distances from the center of injury in surviving TBI patients and in relation to control human cortex. These quantitative observations may be useful in guiding the translation of mitochondrial-based neuroprotective interventions to clinical implementation.
Figures











Similar articles
-
Mitochondrial bioenergetic alterations after focal traumatic brain injury in the immature brain.Exp Neurol. 2015 Sep;271:136-44. doi: 10.1016/j.expneurol.2015.05.009. Epub 2015 May 28. Exp Neurol. 2015. PMID: 26028309 Free PMC article.
-
Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus.J Cereb Blood Flow Metab. 2003 Feb;23(2):219-31. doi: 10.1097/01.WCB.0000040581.43808.03. J Cereb Blood Flow Metab. 2003. PMID: 12571453
-
Traumatic brain injury and mitochondrial dysfunction.Am J Med Sci. 2015 Aug;350(2):132-8. doi: 10.1097/MAJ.0000000000000506. Am J Med Sci. 2015. PMID: 26083647 Review.
-
Quantitative detection of the expression of mitochondrial cytochrome c oxidase subunits mRNA in the cerebral cortex after experimental traumatic brain injury.Brain Res. 2009 Jan 28;1251:287-95. doi: 10.1016/j.brainres.2008.11.034. Epub 2008 Nov 21. Brain Res. 2009. PMID: 19063873
-
The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI.Exp Neurol. 2009 Aug;218(2):363-70. doi: 10.1016/j.expneurol.2009.05.026. Epub 2009 May 27. Exp Neurol. 2009. PMID: 19481077 Review.
Cited by
-
Acute axon damage and demyelination are mitigated by 4-aminopyridine (4-AP) therapy after experimental traumatic brain injury.Acta Neuropathol Commun. 2022 May 2;10(1):67. doi: 10.1186/s40478-022-01366-z. Acta Neuropathol Commun. 2022. PMID: 35501931 Free PMC article.
-
Dynamin and reverse-mode sodium calcium exchanger blockade confers neuroprotection from diffuse axonal injury.Cell Death Dis. 2019 Sep 27;10(10):727. doi: 10.1038/s41419-019-1908-3. Cell Death Dis. 2019. PMID: 31562294 Free PMC article.
-
Can Ultrasound-Guided Xenon Delivery Provide Neuroprotection in Traumatic Brain Injury?Neurotrauma Rep. 2022 Feb 22;3(1):97-104. doi: 10.1089/neur.2021.0070. eCollection 2022. Neurotrauma Rep. 2022. PMID: 35317306 Free PMC article.
-
Traumatic Brain Injury and Coenzyme Q10: An Overview.Int J Mol Sci. 2025 May 27;26(11):5126. doi: 10.3390/ijms26115126. Int J Mol Sci. 2025. PMID: 40507939 Free PMC article. Review.
-
Mitochondrial bioenergetic alterations after focal traumatic brain injury in the immature brain.Exp Neurol. 2015 Sep;271:136-44. doi: 10.1016/j.expneurol.2015.05.009. Epub 2015 May 28. Exp Neurol. 2015. PMID: 26028309 Free PMC article.
References
-
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J. Neurotrauma. 2000;17:843–855. - PubMed
-
- Lifshitz J. Sullivan P.G. Hovda D.A. Wieloch T. McIntosh T.K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. - PubMed
-
- Okonkwo D.O. Povlishock J.T. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 1999;19:443–451. - PubMed
-
- Bereiter-Hahn J. Jendrach M. Mitochondrial dynamics. Int. Rev. Cell Mol. Biol. 2010;284:1–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

