Adaptation of a web-based, open source electronic medical record system platform to support a large study of tuberculosis epidemiology
- PMID: 23131180
- PMCID: PMC3531253
- DOI: 10.1186/1472-6947-12-125
Adaptation of a web-based, open source electronic medical record system platform to support a large study of tuberculosis epidemiology
Abstract
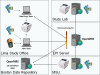
Background: In 2006, we were funded by the US National Institutes of Health to implement a study of tuberculosis epidemiology in Peru. The study required a secure information system to manage data from a target goal of 16,000 subjects who needed to be followed for at least one year. With previous experience in the development and deployment of web-based medical record systems for TB treatment in Peru, we chose to use the OpenMRS open source electronic medical record system platform to develop the study information system. Supported by a core technical and management team and a large and growing worldwide community, OpenMRS is now being used in more than 40 developing countries. We adapted the OpenMRS platform to better support foreign languages. We added a new module to support double data entry, linkage to an existing laboratory information system, automatic upload of GPS data from handheld devices, and better security and auditing of data changes. We added new reports for study managers, and developed data extraction tools for research staff and statisticians. Further adaptation to handle direct entry of laboratory data occurred after the study was launched.
Results: Data collection in the OpenMRS system began in September 2009. By August 2011 a total of 9,256 participants had been enrolled, 102,274 forms and 13,829 laboratory results had been entered, and there were 208 users. The system is now entirely supported by the Peruvian study staff and programmers.
Conclusions: The information system served the study objectives well despite requiring some significant adaptations mid-stream. OpenMRS has more tools and capabilities than it did in 2008, and requires less adaptations for future projects. OpenMRS can be an effective research data system in resource poor environments, especially for organizations using or considering it for clinical care as well as research.
Figures
References
-
- Espinal MA, Laszlo A, Simonsen L. et al.Global trends in resistance to anti-tuberculosis drugs. World Health Organization– International Union against tuberculosis and lung disease working group on anti-tuberculosis drug resistance surveillance. N Engl J Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. - DOI - PubMed
-
- Mitnick CD, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, Fitzmaurice GM, Alcántara F, Appleton SC, Bonilla C, Chalco K, Choi S, Franke MF, Fraser HSF, Guerra D, Hurtado RM, Jazayeri D, Joseph K, Llaro K, Mestanza L, Mukherjee JS, Muñoz M, Palacios E, Sánchez E, Bayona J, Becerra MC. Extensively Drug-Resistant Tuberculosis: A Comprehensive Treatment Approach. N Engl J Med. 2008;359(Suppl 6):563–574. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources