Functional diversity of human vaginal APC subsets in directing T-cell responses
- PMID: 23131784
- PMCID: PMC3568194
- DOI: 10.1038/mi.2012.104
Functional diversity of human vaginal APC subsets in directing T-cell responses
Abstract
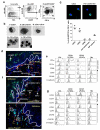
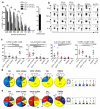
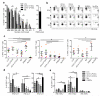
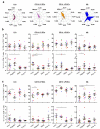
Human vaginal mucosa is the major entry site of sexually transmitted pathogens and thus has long been attractive as a site for mounting mucosal immunity. It is also known as a tolerogenic microenvironment. Here, we demonstrate that immune responses in the vagina can be orchestrated by the functional diversity of four major antigen-presenting cell (APC) subsets. Langerhans cells (LCs) and CD14(-) lamina propria-dendritic cells (LP-DCs) polarize CD4(+) and CD8(+) T cells toward T-helper type 2 (Th2), whereas CD14(+) LP-DCs and macrophages polarize CD4(+) T cells toward Th1. Both LCs and CD14(-) LP-DCs are potent inducers of Th22. Owing to their functional specialties and the different expression levels of pattern-recognition receptors on the APC subsets, microbial products do not bias them to elicit common types of immune responses (Th1 or Th2). To evoke desired types of adaptive immune responses in the human vagina, antigens may need to be targeted to proper APC subsets with right adjuvants.
Figures


References
-
- Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53(5):208–214. - PubMed
-
- Russell MW, Mestecky J. Tolerance and protection against infection in the genital tract. Immunol Invest. 2010;39(4-5):500–525. - PubMed
-
- Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4(6):667–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

