Novel Phosphorylations of IKKγ/NEMO
- PMID: 23131831
- PMCID: PMC3487776
- DOI: 10.1128/mBio.00411-12
Novel Phosphorylations of IKKγ/NEMO
Abstract
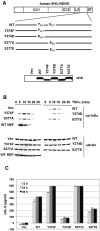
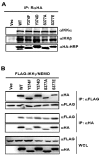
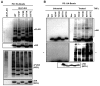
Central to NF-κB signaling pathways is IKKγ/NEMO, a regulatory subunit of the cytoplasmic IκB kinase (IKK) complex, which undergoes various posttranslational modifications, specifically phosphorylation, to regulate its function. Furthermore, Kaposi's sarcoma-associated herpesvirus (KSHV) FADD-like interleukin-1β (IL-1β) converting enzyme (FLICE) inhibitory protein (vFLIP) activates the NF-κB signaling pathway by directly interacting with IKKγ/NEMO. However, the exact functions of IKKγ/NEMO phosphorylation and its KvFLIP interaction in NF-κB activation remain elusive. Here, we report two novel phosphorylation sites of IKKγ/NEMO and their negative effect on the IKKγ/NEMO-mediated NF-κB signaling pathway. First, the Src family protein tyrosine kinases (SF-PTKs), including Src, Fyn, Lyn, and Fgr, interact with and phosphorylate tyrosine residue 374 (Y374) of IKKγ/NEMO. Mutation of the Y374 residue to phenylalanine (Y374F) specifically abolished SF-PTK-mediated tyrosine phosphorylation, leading to increased tumor necrosis factor alpha (TNF-α)-induced NF-κB activity. Moreover, our mass spectrometry analysis found that the serine 377 residue (S377) of IKKγ/NEMO underwent robust phosphorylation upon KvFLIP expression. Replacement of the IKKγ/NEMO S377 residue by alanine (S377A) or glutamic acid (S377E) resulted in a significant increase or decrease of NF-κB activity and TNF-α-mediated IL-6 cytokine production, respectively. Our study thus demonstrates that the Y374 or S377 residue located at the C-terminal proline-rich domain of human IKKγ/NEMO undergoes phosphorylation upon TNF-α treatment or KvFLIP expression, respectively, resulting in the suppression of IKKγ/NEMO activity to induce NF-κB activation. This study suggests the potential phosphorylation-mediated feedback negative regulation of IKKγ/NEMO activity in the NF-κB signaling pathway. IMPORTANCE Since unchecked regulation of NF-κB has been linked to uncontrolled proliferation and cell death, the downregulation of the NF-κB signaling pathway is as important as its activation. Specifically, the phosphorylation-mediated modification of IKKγ/NEMO is a critical regulatory mechanism of NF-κB activity. Here, we report two novel phosphorylations of IKKγ/NEMO and their negative effects on the NF-κB signaling pathway. First, the Src family protein tyrosine kinase interacts with and phosphorylates tyrosine residue 374 of IKKγ/NEMO, suppressing tumor necrosis factor alpha (TNF-α)-induced NF-κB activity. Additionally, Kaposi's sarcoma-associated herpesvirus (KSHV) FADD-like interleukin-1β (IL-1β) converting enzyme (FLICE) inhibitory protein (KvFLIP) expression induces a robust phosphorylation of the serine 377 residue of IKKγ/NEMO, resulting in a significant decrease of NF-κB activity. Our study thus demonstrates that the Y374 or S377 residue of IKKγ/NEMO undergoes phosphorylation upon TNF-α treatment or KvFLIP expression, respectively, resulting in the suppression of IKKγ/NEMO activity to induce NF-κB activation. This also suggests the potential phosphorylation-mediated feedback negative regulation of IKKγ/NEMO activity in the NF-κB signaling pathway.
Figures


Similar articles
-
NEMO is essential for Kaposi's sarcoma-associated herpesvirus-encoded vFLIP K13-induced gene expression and protection against death receptor-induced cell death, and its N-terminal 251 residues are sufficient for this process.J Virol. 2014 Jun;88(11):6345-54. doi: 10.1128/JVI.00028-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672029 Free PMC article.
-
Protein Kinase-Mediated Decision Between the Life and Death.Adv Exp Med Biol. 2021;1275:1-33. doi: 10.1007/978-3-030-49844-3_1. Adv Exp Med Biol. 2021. PMID: 33539010
-
Kaposi's sarcoma associated herpesvirus encoded viral FLICE inhibitory protein K13 activates NF-κB pathway independent of TRAF6, TAK1 and LUBAC.PLoS One. 2012;7(5):e36601. doi: 10.1371/journal.pone.0036601. Epub 2012 May 8. PLoS One. 2012. PMID: 22590573 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Protein-protein interactions involving IKKgamma (NEMO) that promote the activation of NF-kappaB.J Cell Physiol. 2010 Jun;223(3):558-61. doi: 10.1002/jcp.22105. J Cell Physiol. 2010. PMID: 20301198 Review.
Cited by
-
The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation.J Exp Med. 2014 Jun 30;211(7):1333-47. doi: 10.1084/jem.20132486. Epub 2014 Jun 23. J Exp Med. 2014. PMID: 24958845 Free PMC article.
-
A conserved core region of the scaffold NEMO is essential for signal-induced conformational change and liquid-liquid phase separation.J Biol Chem. 2023 Dec;299(12):105396. doi: 10.1016/j.jbc.2023.105396. Epub 2023 Oct 27. J Biol Chem. 2023. PMID: 37890781 Free PMC article.
-
The role of the IKK complex in viral infections.Pathog Dis. 2014 Oct;72(1):32-44. doi: 10.1111/2049-632X.12210. Epub 2014 Aug 28. Pathog Dis. 2014. PMID: 25082354 Free PMC article. Review.
-
A Conserved Core Region of the Scaffold NEMO is Essential for Signal-induced Conformational Change and Liquid-liquid Phase Separation.bioRxiv [Preprint]. 2023 May 25:2023.05.25.542299. doi: 10.1101/2023.05.25.542299. bioRxiv. 2023. Update in: J Biol Chem. 2023 Dec;299(12):105396. doi: 10.1016/j.jbc.2023.105396. PMID: 37292615 Free PMC article. Updated. Preprint.
-
The Role of vIL-6 in KSHV-Mediated Immune Evasion and Tumorigenesis.Viruses. 2024 Dec 10;16(12):1900. doi: 10.3390/v16121900. Viruses. 2024. PMID: 39772207 Free PMC article. Review.
References
-
- Häcker H, Karin M. 2006. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13. http://dx.doi.org/10.1126/stke.3572006re13. - PubMed
-
- Vallabhapurapu S, Karin M. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27:693–733 - PubMed
-
- Israël A. 2010. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harbor Perspect. Biol. 2:a000158 http://dx.doi.org/10.1101/cshperspect.a000158 - PMC - PubMed
-
- Ruland J. 2011. Return to homeostasis: downregulation of NF-kappaB responses. Nat. Immunol. 12:709–714 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

