Grb2, a double-edged sword of receptor tyrosine kinase signaling
- PMID: 23131845
- PMCID: PMC3668340
- DOI: 10.1126/scisignal.2003576
Grb2, a double-edged sword of receptor tyrosine kinase signaling
Abstract
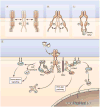
Receptor tyrosine kinases (RTKs) exhibit basal tyrosine phosphorylation and activity in the absence of ligand stimulation, which has been attributed to the "leaky" nature of tyrosine kinase autoinhibition and stochastic collisions of receptors in the membrane bilayer. This basal phosphorylation does not produce a signal of sufficient amplitude and intensity to manifest in a biological response and hence is considered to be a passive, futile process that does not have any biological function. This paradigm has now been challenged by a study showing that the basal phosphorylation of RTKs is a physiologically relevant process that is actively inhibited by the intracellular adaptor protein growth factor receptor-bound 2 (Grb2) and serves to "prime" receptors for a rapid response to ligand stimulation. Grb2 is conventionally known for playing positive roles in RTK signaling. The discovery of a negative regulatory role for Grb2 reveals that this adaptor acts as a double-edged sword in the regulation of RTK signaling.
Figures
Similar articles
-
Distinct involvement of the Gab1 and Grb2 adaptor proteins in signal transduction by the related receptor tyrosine kinases RON and MET.J Biol Chem. 2011 Sep 16;286(37):32762-74. doi: 10.1074/jbc.M111.239384. Epub 2011 Jul 22. J Biol Chem. 2011. PMID: 21784853 Free PMC article.
-
Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase.J Biol Chem. 2000 Dec 15;275(50):39159-66. doi: 10.1074/jbc.M006908200. J Biol Chem. 2000. PMID: 10995764
-
Signaling adaptor ShcD suppresses extracellular signal-regulated kinase (Erk) phosphorylation distal to the Ret and Trk neurotrophic receptors.J Biol Chem. 2017 Apr 7;292(14):5748-5759. doi: 10.1074/jbc.M116.770511. Epub 2017 Feb 17. J Biol Chem. 2017. PMID: 28213521 Free PMC article.
-
Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases.Trends Cell Biol. 2001 Jun;11(6):258-66. doi: 10.1016/s0962-8924(01)01990-0. Trends Cell Biol. 2001. PMID: 11356362 Review.
-
Endocytosis of receptor tyrosine kinases.Cold Spring Harb Perspect Biol. 2013 May 1;5(5):a017459. doi: 10.1101/cshperspect.a017459. Cold Spring Harb Perspect Biol. 2013. PMID: 23637288 Free PMC article. Review.
Cited by
-
Negative regulation of receptor tyrosine kinases by ubiquitination: Key roles of the Cbl family of E3 ubiquitin ligases.Front Endocrinol (Lausanne). 2022 Jul 28;13:971162. doi: 10.3389/fendo.2022.971162. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35966060 Free PMC article. Review.
-
Structure of FGFR3 transmembrane domain dimer: implications for signaling and human pathologies.Structure. 2013 Nov 5;21(11):2087-93. doi: 10.1016/j.str.2013.08.026. Epub 2013 Oct 10. Structure. 2013. PMID: 24120763 Free PMC article.
-
DDR1 promotes metastasis of cervical cancer and downstream phosphorylation signal via binding GRB2.Cell Death Dis. 2024 Nov 20;15(11):849. doi: 10.1038/s41419-024-07212-5. Cell Death Dis. 2024. PMID: 39567474 Free PMC article.
-
Extensive rewiring of the EGFR network in colorectal cancer cells expressing transforming levels of KRASG13D.Nat Commun. 2020 Jan 24;11(1):499. doi: 10.1038/s41467-019-14224-9. Nat Commun. 2020. PMID: 31980649 Free PMC article.
-
VEGFR-2 conformational switch in response to ligand binding.Elife. 2016 Apr 7;5:e13876. doi: 10.7554/eLife.13876. Elife. 2016. PMID: 27052508 Free PMC article.
References
-
- Stroud RM, Wells JA. Mechanistic diversity of cytokine receptor signaling across cell membranes. Sci STKE. 2004;2004:re7. - PubMed
-
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

