Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells
- PMID: 23132245
- PMCID: PMC3509731
- DOI: 10.1242/dev.083840
Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells
Abstract
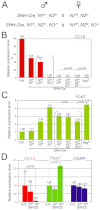
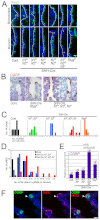
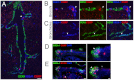
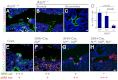
In the developing lung, it is thought that the terminal buds of elongating airways contain a population of multipotent epithelial progenitors. As the bronchial tree extends, descendants of these cells give rise to lineage-restricted progenitors in the conducting airways via Notch signaling, which is involved in the establishment of epithelial Clara, ciliated and pulmonary neuroendocrine (NE) cell populations. However, the precise molecular details of this selection process are still emerging. Our stepwise removal of the three Notch receptors from the developing lung epithelium reveals that, whereas Notch2 mediates the Clara/ciliated cell fate decision with negligible contributions from Notch1 and Notch3, all three Notch receptors contribute in an additive manner to regulate the abundance of NE cells and the size of the presumptive pulmonary neuroepithelial body (pNEB) as a result of mutual interactions between NE cells and the Notch-dependent, SSEA-1(+), CC10(-) cell population surrounding the pNEB (SPNC cells). Ectopic expression of the Notch1 or Notch2 intracellular domain was sufficient to induce SSEA-1(+) cells and to suppress pNEB formation without expending Clara cells. We provide evidence that the additive functions of Notch receptors, together with other signaling pathways, maintains the expression of Hes1, a key regulator of NE cell fate, and that maintenance of Hes1 expression in epithelial cells is key to the regulation of pNEB size. These results suggest that two different assemblies of Notch receptors coordinate the numbers and distribution of the major epithelial cell types in the conducting airway during lung organogenesis.
Figures
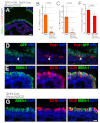
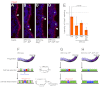
References
-
- Borges M., Linnoila R. I., van de Velde H. J., Chen H., Nelkin B. D., Mabry M., Baylin S. B., Ball D. W. (1997). An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 386, 852-855 - PubMed
-
- Cardoso W. V., Lü J. (2006). Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611-1624 - PubMed
-
- Guha A., Vasconcelos M., Cai Y., Yoneda M., Hinds A., Qian J., Li G., Dickel L., Johnson J. E., Kimura S., et al. (2012). Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc. Natl. Acad. Sci. USA 109, 12592-12597 - PMC - PubMed
-
- Guillemot F., Lo L. C., Johnson J. E., Auerbach A., Anderson D. J., Joyner A. L. (1993). Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75, 463-476 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

