Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway : scriptaid protects against TBI via AKT
- PMID: 23132328
- PMCID: PMC3557358
- DOI: 10.1007/s13311-012-0157-2
Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway : scriptaid protects against TBI via AKT
Abstract
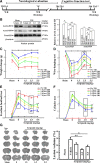
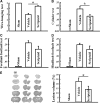
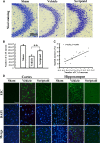
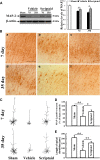
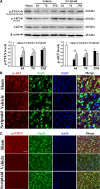
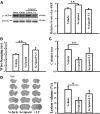
Traumatic brain injury (TBI) is a leading cause of motor and cognitive deficits in young adults for which there is no effective therapy. The present study characterizes the protective effect of a new histone deacetylase inhibitor, Scriptaid (Sigma-Aldrich Corporation, St. Louis, MO), against injury from controlled cortical impact (CCI). Scriptaid elicited a dose-dependent decrease in lesion size at 1.5 to 5.5 mg/kg and a concomitant attenuation in motor and cognitive deficits when delivered 30 minutes postinjury in a model of moderate TBI. Comparable protection was achieved even when treatment was delayed to 12 h postinjury. Furthermore, the protection of motor and cognitive functions was long lasting, as similar improvements were detected 35 days postinjury. The efficacy of Scriptaid (Sigma-Aldrich Corporation) was manifested as an increase in surviving neurons, as well as the number/length of their processes within the CA3 region of the hippocampus and the pericontusional cortex. Consistent with other histone deacetylase inhibitors, Scriptaid treatment prevented the decrease in phospho-AKT (p-AKT) and phosphorylated phosphatase and tensin homolog deleted on chromosome 10 (p-PTEN) induced by TBI in cortical and CA3 hippocampal neurons. Notably, the p-AKT inhibitor LY294002 attenuated the impact of Scriptaid, providing mechanistic evidence that Scriptaid functions partly by modulating the prosurvival AKT signaling pathway. As Scriptaid offers long-lasting neuronal and behavioral protection, even when delivered 12 h after controlled cortical impact, it is an excellent new candidate for the effective clinical treatment of TBI.
Figures


Similar articles
-
HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2853-8. doi: 10.1073/pnas.1501441112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25691750 Free PMC article.
-
Histone Deacetylase Inhibitor Scriptaid Alleviated Neurological Dysfunction after Experimental Intracerebral Hemorrhage in Mice.Behav Neurol. 2018 Aug 12;2018:6583267. doi: 10.1155/2018/6583267. eCollection 2018. Behav Neurol. 2018. PMID: 30159100 Free PMC article.
-
Inhibition of phosphatase and tensin homolog deleted on chromosome 10 decreases rat cortical neuron injury and blood-brain barrier permeability, and improves neurological functional recovery in traumatic brain injury model.PLoS One. 2013 Nov 28;8(11):e80429. doi: 10.1371/journal.pone.0080429. eCollection 2013. PLoS One. 2013. PMID: 24312220 Free PMC article.
-
miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury.Brain Res. 2014 Sep 25;1582:12-20. doi: 10.1016/j.brainres.2014.07.045. Epub 2014 Aug 6. Brain Res. 2014. PMID: 25108037
-
Pharmacokinetics and Acute Toxicity of a Histone Deacetylase Inhibitor, Scriptaid, and its Neuroprotective Effects in Mice After Intracranial Hemorrhage.CNS Neurol Disord Drug Targets. 2020;19(1):55-65. doi: 10.2174/1871527319666191220111126. CNS Neurol Disord Drug Targets. 2020. PMID: 31858907
Cited by
-
HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2853-8. doi: 10.1073/pnas.1501441112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25691750 Free PMC article.
-
Brain-selective mild hypothermia promotes long-term white matter integrity after ischemic stroke in mice.CNS Neurosci Ther. 2018 Dec;24(12):1275-1285. doi: 10.1111/cns.13061. Epub 2018 Sep 16. CNS Neurosci Ther. 2018. PMID: 30295998 Free PMC article.
-
Effects of deferoxamine on blood-brain barrier disruption after subarachnoid hemorrhage.PLoS One. 2017 Mar 1;12(3):e0172784. doi: 10.1371/journal.pone.0172784. eCollection 2017. PLoS One. 2017. PMID: 28249040 Free PMC article.
-
White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke.Exp Neurol. 2015 Oct;272:109-19. doi: 10.1016/j.expneurol.2015.03.021. Epub 2015 Mar 31. Exp Neurol. 2015. PMID: 25836044 Free PMC article.
-
RNA Interference Unleashed: Current Perspective of Small Interfering RNA (siRNA) Therapeutics in the Treatment of Neuropathic Pain.ACS Pharmacol Transl Sci. 2024 Sep 23;7(10):2951-2970. doi: 10.1021/acsptsci.4c00329. eCollection 2024 Oct 11. ACS Pharmacol Transl Sci. 2024. PMID: 39416962 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

