Opioid analgesia in mechanically ventilated children: results from the multicenter Measuring Opioid Tolerance Induced by Fentanyl study
- PMID: 23132396
- PMCID: PMC3581608
- DOI: 10.1097/PCC.0b013e318253c80e
Opioid analgesia in mechanically ventilated children: results from the multicenter Measuring Opioid Tolerance Induced by Fentanyl study
Abstract
Objective: To examine the clinical factors associated with increased opioid dose among mechanically ventilated children in the pediatric intensive care unit.
Design: Prospective, observational study with 100% accrual of eligible patients.
Setting: Seven pediatric intensive care units from tertiary-care children's hospitals in the Collaborative Pediatric Critical Care Research Network.
Patients: Four hundred nineteen children treated with morphine or fentanyl infusions.
Interventions: None.
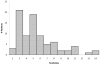
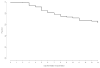
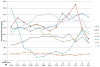
Measurements and main results: Data on opioid use, concomitant therapy, demographic and explanatory variables were collected. Significant variability occurred in clinical practices, with up to 100-fold differences in baseline opioid doses, average daily or total doses, or peak infusion rates. Opioid exposure for 7 or 14 days required doubling of the daily opioid dose in 16% patients (95% confidence interval 12%-19%) and 20% patients (95% confidence interval 16%-24%), respectively. Among patients receiving opioids for longer than 3 days (n = 225), this occurred in 28% (95% confidence interval 22%-33%) and 35% (95% confidence interval 29%-41%) by 7 or 14 days, respectively. Doubling of the opioid dose was more likely to occur following opioid infusions for 7 days or longer (odds ratio 7.9, 95% confidence interval 4.3-14.3; p < 0.001) or co-therapy with midazolam (odds ratio 5.6, 95% confidence interval 2.4-12.9; p < 0.001), and it was less likely to occur if morphine was used as the primary opioid (vs. fentanyl) (odds ratio 0.48, 95% confidence interval 0.25-0.92; p = 0.03), for patients receiving higher initial doses (odds ratio 0.96, 95% confidence interval 0.95-0.98; p < 0.001), or if patients had prior pediatric intensive care unit admissions (odds ratio 0.37, 95% confidence interval 0.15-0.89; p = 0.03).
Conclusions: Mechanically ventilated children require increasing opioid doses, often associated with prolonged opioid exposure or the need for additional sedation. Efforts to reduce prolonged opioid exposure and clinical practice variation may prevent the complications of opioid therapy.
Conflict of interest statement
Figures
Comment in
-
Analgesia in mechanically ventilated children: to each his own?Pediatr Crit Care Med. 2013 Jan;14(1):101-2. doi: 10.1097/PCC.0b013e31825b87d8. Pediatr Crit Care Med. 2013. PMID: 23295838 No abstract available.
References
-
- Jenkins IA, Playfor SD, Bevan C, et al. Current United Kingdom sedation practice in pediatric intensive care. Paediatr Anaesth. 2007;17(7):675–683. - PubMed
-
- Tobias JD. Sedation and analgesia in the pediatric intensive care unit. Pediatr Ann. 2005;34(8):636–645. - PubMed
-
- Anand KJS, Aranda JV, Berde CB, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006;117(3 Pt 2):S9–S22. - PubMed
-
- Anand KJS. Relationships between stress responses and clinical outcome in newborns, infants, and children. Critical Care Medicine. 1993;21(9 Suppl):S358–359. - PubMed
-
- Simons SH, Anand KJS. Pain control: opioid dosing, population kinetics and side-effects. Semin Fetal Neonatal Med. 2006;11(4):260–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10HD049945/HD/NICHD NIH HHS/United States
- U10 HD050012/HD/NICHD NIH HHS/United States
- U10 HD050009/HD/NICHD NIH HHS/United States
- U10 HD049945/HD/NICHD NIH HHS/United States
- U10HD049981/HD/NICHD NIH HHS/United States
- UG1 HD050096/HD/NICHD NIH HHS/United States
- U10 HD049981/HD/NICHD NIH HHS/United States
- U10 HD050096/HD/NICHD NIH HHS/United States
- U10 HD049983/HD/NICHD NIH HHS/United States
- RL1 HD107773/HD/NICHD NIH HHS/United States
- U10HD050096/HD/NICHD NIH HHS/United States
- U10HD049983/HD/NICHD NIH HHS/United States
- U10HD050012/HD/NICHD NIH HHS/United States
- U01HD049934/HD/NICHD NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U01 HD049934/HD/NICHD NIH HHS/United States
- U10HD500009/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

