Aldehyde dehydrogenase 1A1--a new mediator of resistance to temozolomide in glioblastoma
- PMID: 23132408
- PMCID: PMC3499020
- DOI: 10.1093/neuonc/nos270
Aldehyde dehydrogenase 1A1--a new mediator of resistance to temozolomide in glioblastoma
Abstract
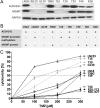
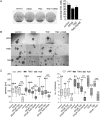
Implementation of chemotherapy with the drug temozolomide increased the overall survival of patients with glioblastoma multiforme (GBM; WHO grade IV), in particular when the O(6)-methylguanine DNA methyltransferase (MGMT) promoter is epigenetically silenced. Nevertheless, the prognosis remains poor, and relapse in GBM occurs regularly. This clinical behavior seems to be due to the existence of a therapy-resistant subpopulation of cells that induce tumor regrowth. The objective of this work was to analyze the role of aldehyde dehydrogenase (ALDH) 1A1 in mediating temozolomide resistance and its value as a predictor of clinical outcome in GBM patients. Nine GBM cell lines were treated with temozolomide alone or in combination with 4-diethylaminobenzaldehyde (DEAB), an inhibitor of ALDH1A1, or with ALDH1A1 short hairpin (sh)RNA. ALDH1A1 expression and MGMT status of 70 primary GBM patients were correlated with median survival. ALDH1A1 overexpression predicted temozolomide resistance in vitro. Sensitivity of ALDH1A1 positive/MGMT-positive cells to temozolomide could be restored by inhibition of ALDH1A1 by DEAB or by knockdown with shRNA, as indicated by increased cytotoxicity, reduced clonogenicity, and accumulation in the G2/M cell-cycle phase. The prognosis of patients with a high level of ALDH1A1 expression was poor compared with that of patients with low levels (P < .0001). ALDH1A1 is a new mediator for resistance of GBM to temozolomide and a reliable predictor of clinical outcome and may serve as a potential target to improve treatment of human GBM.
Figures



Similar articles
-
MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma.Pediatr Blood Cancer. 2007 Apr;48(4):403-7. doi: 10.1002/pbc.20803. Pediatr Blood Cancer. 2007. PMID: 16609952
-
Inhibition of histone deacetylation potentiates the evolution of acquired temozolomide resistance linked to MGMT upregulation in glioblastoma xenografts.Clin Cancer Res. 2012 Aug 1;18(15):4070-9. doi: 10.1158/1078-0432.CCR-12-0560. Epub 2012 Jun 6. Clin Cancer Res. 2012. PMID: 22675172 Free PMC article.
-
O6-Methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients.Neuro Oncol. 2010 Jan;12(1):28-36. doi: 10.1093/neuonc/nop003. Epub 2009 Oct 15. Neuro Oncol. 2010. PMID: 20150365 Free PMC article.
-
The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials.J Cell Physiol. 2018 Jan;233(1):378-386. doi: 10.1002/jcp.25896. Epub 2017 May 16. J Cell Physiol. 2018. PMID: 28266716 Review.
-
The prognostic value of MGMT promoter status by pyrosequencing assay for glioblastoma patients' survival: a meta-analysis.World J Surg Oncol. 2016 Oct 12;14(1):261. doi: 10.1186/s12957-016-1012-4. World J Surg Oncol. 2016. PMID: 27733166 Free PMC article. Review.
Cited by
-
β-escin selectively targets the glioblastoma-initiating cell population and reduces cell viability.Oncotarget. 2016 Oct 11;7(41):66865-66879. doi: 10.18632/oncotarget.11784. Oncotarget. 2016. PMID: 27589691 Free PMC article.
-
Lipid Peroxidation Plays an Important Role in Chemotherapeutic Effects of Temozolomide and the Development of Therapy Resistance in Human Glioblastoma.Transl Oncol. 2020 Mar;13(3):100748. doi: 10.1016/j.tranon.2020.100748. Epub 2020 Feb 19. Transl Oncol. 2020. PMID: 32087559 Free PMC article.
-
Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells.Br J Cancer. 2013 Oct 1;109(7):1876-85. doi: 10.1038/bjc.2013.534. Epub 2013 Sep 5. Br J Cancer. 2013. PMID: 24008666 Free PMC article.
-
Expression Profile of Genes Related to Drug Metabolism in Human Brain Tumors.PLoS One. 2015 Nov 18;10(11):e0143285. doi: 10.1371/journal.pone.0143285. eCollection 2015. PLoS One. 2015. PMID: 26580399 Free PMC article.
-
Modulating glioblastoma chemotherapy response: Evaluating long non-coding RNA effects on DNA damage response, glioma stem cell function, and hypoxic processes.Neurooncol Adv. 2022 Aug 10;4(1):vdac119. doi: 10.1093/noajnl/vdac119. eCollection 2022 Jan-Dec. Neurooncol Adv. 2022. PMID: 36105389 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

