Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury
- PMID: 23132593
- PMCID: PMC3580267
- DOI: 10.1002/stem.1275
Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury
Abstract
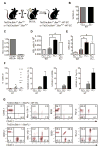
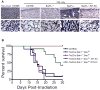
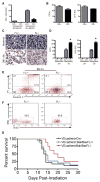
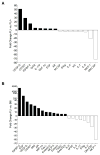
Hematopoietic stem cells (HSCs) reside in proximity to bone marrow endothelial cells (BM ECs) and maintenance of the HSC pool is dependent upon EC-mediated c-kit signaling. Here, we used genetic models to determine whether radioprotection of BM ECs could facilitate hematopoietic regeneration following radiation-induced myelosuppression. We developed mice bearing deletion of the proapoptotic proteins, BAK and BAX, in Tie2(+) ECs and HSCs (Tie2Bak/Bax(Fl/-) mice) and compared their hematopoietic recovery following total body irradiation (TBI) with mice which retained Bax in Tie2(+) cells. Mice bearing deletion of Bak and Bax in Tie2(+) cells demonstrated protection of BM HSCs, preserved BM vasculature, and 100% survival following lethal dose TBI. In contrast, mice that retained Bax expression in Tie2(+) cells demonstrated depletion of BM HSCs, disrupted BM vasculature, and 10% survival post-TBI. In a complementary study, VEcadherinBak/Bax(Fl/-) mice, which lack Bak and Bax in VEcadherin(+) ECs, also demonstrated increased recovery of BM stem/progenitor cells following TBI compared to mice which retained Bax in VEcadherin(+) ECs. Importantly, chimeric mice that lacked Bak and Bax in HSCs but retained Bak and Bax in BM ECs displayed significantly decreased HSC content and survival following TBI compared to mice lacking Bak and Bax in both HSCs and BM ECs. These data suggest that the hematopoietic response to ionizing radiation is dependent upon HSC-autonomous responses but is regulated by BM EC-mediated mechanisms. Therefore, BM ECs may be therapeutically targeted as a means to augment hematopoietic reconstitution following myelosuppression.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
Figures


References
-
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
-
- Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. - PubMed
-
- Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. - PubMed
-
- Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

