Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury
- PMID: 23132743
- PMCID: PMC3529380
- DOI: 10.1210/en.2012-1579
Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury
Abstract
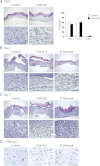
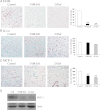
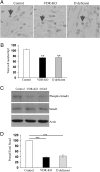
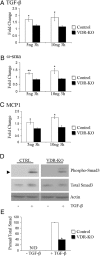
The vitamin D receptor (VDR) has both 1,25-dihydroxyvitamin D-dependent and -independent actions in the epidermis. Ligand-dependent actions of the VDR have been shown to promote keratinocyte differentiation and to regulate formation of the epidermal barrier. In contrast, the actions of the VDR that regulate postmorphogenic hair cycling do not require 1,25-dihydroxyvitamin D. The VDR also has immunomodulatory actions that are dependent on its ligand, 1,25-dihydroxyvitamin D. To determine whether the ligand-dependent or -independent actions of the VDR regulate the inflammatory response to cutaneous injury, studies were performed in control, VDR knockout, and vitamin D-deficient mice. These investigations demonstrate that absence of receptor or ligand impairs the dermal response to cutaneous injury. Although neutrophil recruitment is not affected, the absence of VDR signaling leads to defects in macrophage recruitment and granulation tissue formation. Studies performed to identify the molecular basis for this phenotype demonstrate that absence of the VDR, or its ligand, impairs TGF-β signaling in the dermis, characterized by decreased expression of monocyte chemotactic protein-1 and reduced phosphorylation of phosphorylated Smad-3 as well as attenuated phosphorylated Smad-3 phosphorylation in response to TGF-β in primary dermal fibroblasts lacking the VDR. Thus, these data demonstrate that the liganded VDR interacts with the TGF-β signaling pathway to promote the normal inflammatory response to cutaneous injury.
Figures
References
-
- Bikle DD, Oda Y, Xie Z. 2004. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J Steroid Biochem Mol Biol 89–90:355–360 - PubMed
-
- Sakai Y, Demay MB. 2000. Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology 141:2043–2049 - PubMed
-
- Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. 2002. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol 118:11–16 - PubMed
-
- Oda Y, Ishikawa MH, Hawker NP, Yun QC, Bikle DD. 2007. Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol 103:776–780 - PubMed
-
- Oda Y, Sihlbom C, Chalkley RJ, Huang L, Rachez C, Chang CP, Burlingame AL, Freedman LP, Bikle DD. 2003. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol Endocrinol (Baltimore, Md) 17:2329–2339 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

