Effective elimination of cancer stem cells by a novel drug combination strategy
- PMID: 23132831
- PMCID: PMC3538380
- DOI: 10.1002/stem.1273
Effective elimination of cancer stem cells by a novel drug combination strategy
Abstract
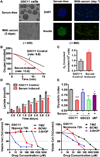
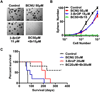
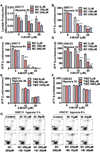
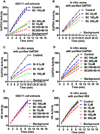
Development of effective therapeutic strategies to eliminate cancer stem cells, which play a major role in drug resistance and disease recurrence, is critical to improve cancer treatment outcomes. Our study showed that glioblastoma stem cells (GSCs) exhibited low mitochondrial respiration and high glycolytic activity. These GSCs were highly resistant to standard drugs such as carmustine and temozolomide (TMZ), but showed high sensitivity to a glycolytic inhibitor 3-bromo-2-oxopropionate-1-propyl ester (3-BrOP), especially under hypoxic conditions. We further showed that combination of 3-BrOP with carmustine but not with TMZ achieved a striking synergistic effect and effectively killed GSCs through a rapid depletion of cellular ATP and inhibition of carmustine-induced DNA repair. This drug combination significantly impaired the sphere-forming ability of GSCs in vitro and tumor formation in vivo, leading to increase in the overall survival of mice bearing orthotopic inoculation of GSCs. Further mechanistic study showed that 3-BrOP and carmustine inhibited glyceraldehyde-3-phosphate dehydrogenase and caused a severe energy crisis in GSCs. Our study suggests that GSCs are highly glycolytic and that certain drug combination strategies can be used to effectively overcome their drug resistance based on their metabolic properties.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures



References
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. - PubMed
-
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. - PubMed
-
- O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

