Disease-associated mutations in the prion protein impair laminin-induced process outgrowth and survival
- PMID: 23132868
- PMCID: PMC3527962
- DOI: 10.1074/jbc.M112.428235
Disease-associated mutations in the prion protein impair laminin-induced process outgrowth and survival
Abstract
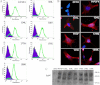
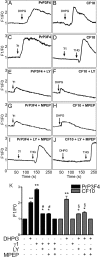
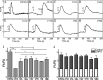
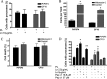
Prions, the agents of transmissible spongiform encephalopathies, require the expression of prion protein (PrP(C)) to propagate disease. PrP(C) is converted into an abnormal insoluble form, PrP(Sc), that gains neurotoxic activity. Conversely, clinical manifestations of prion disease may occur either before or in the absence of PrP(Sc) deposits, but the loss of normal PrP(C) function contribution for the etiology of these diseases is still debatable. Prion disease-associated mutations in PrP(C) represent one of the best models to understand the impact of PrP(C) loss-of-function. PrP(C) associates with various molecules and, in particular, the interaction of PrP(C) with laminin (Ln) modulates neuronal plasticity and memory formation. To assess the functional alterations associated with PrP(C) mutations, wild-type and mutated PrP(C) proteins were expressed in a neural cell line derived from a PrP(C)-null mouse. Treatment with the laminin γ1 chain peptide (Ln γ1), which mimics the Ln binding site for PrP(C), increased intracellular calcium in cells expressing wild-type PrP(C), whereas a significantly lower response was observed in cells expressing mutated PrP(C) molecules. The Ln γ1 did not promote process outgrowth or protect against staurosporine-induced cell death in cells expressing mutated PrP(C) molecules in contrast to cells expressing wild-type PrP(C). The co-expression of wild-type PrP(C) with mutated PrP(C) molecules was able to rescue the Ln protective effects, indicating the lack of negative dominance of PrP(C) mutated molecules. These results indicate that PrP(C) mutations impair process outgrowth and survival mediated by Ln γ1 peptide in neural cells, which may contribute to the pathogenesis of genetic prion diseases.
Figures

Similar articles
-
Metabotropic glutamate receptors transduce signals for neurite outgrowth after binding of the prion protein to laminin γ1 chain.FASEB J. 2011 Jan;25(1):265-79. doi: 10.1096/fj.10-161653. Epub 2010 Sep 27. FASEB J. 2011. PMID: 20876210
-
Regulation of Amyloid β Oligomer Binding to Neurons and Neurotoxicity by the Prion Protein-mGluR5 Complex.J Biol Chem. 2016 Oct 14;291(42):21945-21955. doi: 10.1074/jbc.M116.738286. Epub 2016 Aug 25. J Biol Chem. 2016. PMID: 27563063 Free PMC article.
-
Laminin-γ1 chain and stress inducible protein 1 synergistically mediate PrPC-dependent axonal growth via Ca2+ mobilization in dorsal root ganglia neurons.J Neurochem. 2013 Jan;124(2):210-23. doi: 10.1111/jnc.12091. Epub 2012 Dec 4. J Neurochem. 2013. PMID: 23145988
-
Prion protein: orchestrating neurotrophic activities.Curr Issues Mol Biol. 2010;12(2):63-86. Epub 2009 Sep 18. Curr Issues Mol Biol. 2010. PMID: 19767651 Review.
-
Emerging roles of the cellular prion protein (PrPC) and 37/67 kDa laminin receptor (RPSA) interaction in cancer biology.Cell Mol Life Sci. 2023 Jul 15;80(8):207. doi: 10.1007/s00018-023-04844-2. Cell Mol Life Sci. 2023. PMID: 37452879 Free PMC article. Review.
Cited by
-
Hampering the early aggregation of PrP-E200K protein by charge-based inhibitors: a computational study.J Comput Aided Mol Des. 2021 Jun;35(6):751-770. doi: 10.1007/s10822-021-00393-7. Epub 2021 Jun 10. J Comput Aided Mol Des. 2021. PMID: 34110550 Free PMC article.
-
Mutations in Prion Protein Gene: Pathogenic Mechanisms in C-Terminal vs. N-Terminal Domain, a Review.Int J Mol Sci. 2019 Jul 23;20(14):3606. doi: 10.3390/ijms20143606. Int J Mol Sci. 2019. PMID: 31340582 Free PMC article. Review.
-
Roles of prion proteins in mammalian development.Anim Cells Syst (Seoul). 2024 Dec 10;28(1):551-566. doi: 10.1080/19768354.2024.2436860. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 39664939 Free PMC article. Review.
-
Prion protein facilitates synaptic vesicle release by enhancing release probability.Hum Mol Genet. 2014 Sep 1;23(17):4581-96. doi: 10.1093/hmg/ddu171. Epub 2014 Apr 9. Hum Mol Genet. 2014. PMID: 24722203 Free PMC article.
-
Permselectivity of Silk Fibroin Hydrogels for Advanced Drug Delivery Neurotherapies.Biomacromolecules. 2024 Aug 12;25(8):5233-5250. doi: 10.1021/acs.biomac.4c00629. Epub 2024 Jul 17. Biomacromolecules. 2024. PMID: 39018332 Free PMC article.
References
-
- Weissmann C. (2004) The state of the prion. Nat. Rev. Microbiol. 2, 861–871 - PubMed
-
- Mallucci G., Dickinson A., Linehan J., Klöhn P. C., Brandner S., Collinge J. (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302, 871–874 - PubMed
-
- Mallucci G. R., White M. D., Farmer M., Dickinson A., Khatun H., Powell A. D., Brandner S., Jefferys J. G., Collinge J. (2007) Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 53, 325–335 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

