RNA polymerase III subunit architecture and implications for open promoter complex formation
- PMID: 23132938
- PMCID: PMC3511066
- DOI: 10.1073/pnas.1211665109
RNA polymerase III subunit architecture and implications for open promoter complex formation
Abstract
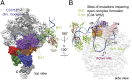
Transcription initiation by eukaryotic RNA polymerase (Pol) III relies on the TFIIE-related subcomplex C82/34/31. Here we combine cross-linking and hydroxyl radical probing to position the C82/34/31 subcomplex around the Pol III active center cleft. The extended winged helix (WH) domains 1 and 4 of C82 localize to the polymerase domains clamp head and clamp core, respectively, and the two WH domains of C34 span the polymerase cleft from the coiled-coil region of the clamp to the protrusion. The WH domains of C82 and C34 apparently cooperate with other mobile regions flanking the cleft during promoter DNA binding, opening, and loading. Together with published data, our results complete the subunit architecture of Pol III and indicate that all TFIIE-related components of eukaryotic and archaeal transcription systems adopt an evolutionarily conserved location in the upper part of the cleft that supports their functions in open promoter complex formation and stabilization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cramer P, et al. Structure of eukaryotic RNA polymerases. Annu Rev Biophys. 2008;37:337–352. - PubMed
-
- Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007;23(12):614–622. - PubMed
-
- Schramm L, Hernandez N. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 2002;16(20):2593–2620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

