Neuronal code for extended time in the hippocampus
- PMID: 23132944
- PMCID: PMC3511087
- DOI: 10.1073/pnas.1214107109
Neuronal code for extended time in the hippocampus
Erratum in
-
Correction for Mankin et al., Neuronal code for extended time in the hippocampus.Proc Natl Acad Sci U S A. 2015 Mar 10;112(10):E1169. doi: 10.1073/pnas.1502758112. Epub 2015 Feb 20. Proc Natl Acad Sci U S A. 2015. PMID: 25713125 Free PMC article. No abstract available.
Abstract
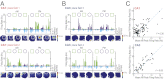
The time when an event occurs can become part of autobiographical memories. In brain structures that support such memories, a neural code should exist that represents when or how long ago events occurred. Here we describe a neuronal coding mechanism in hippocampus that can be used to represent the recency of an experience over intervals of hours to days. When the same event is repeated after such time periods, the activity patterns of hippocampal CA1 cell populations progressively differ with increasing temporal distances. Coding for space and context is nonetheless preserved. Compared with CA1, the firing patterns of hippocampal CA3 cell populations are highly reproducible, irrespective of the time interval, and thus provide a stable memory code over time. Therefore, the neuronal activity patterns in CA1 but not CA3 include a code that can be used to distinguish between time intervals on an extended scale, consistent with behavioral studies showing that the CA1 area is selectively required for temporal coding over such periods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic; 1972. pp. 381–403.
-
- Tulving E. Elements of Episodic Memory. Oxford: Clarendon; 1983.
-
- Friedman WJ. Memory for the time of past events. Psychol Bull. 1993;113(1):44–66.
-
- Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

