MiR-495 is a tumor-suppressor microRNA down-regulated in MLL-rearranged leukemia
- PMID: 23132946
- PMCID: PMC3511140
- DOI: 10.1073/pnas.1217519109
MiR-495 is a tumor-suppressor microRNA down-regulated in MLL-rearranged leukemia
Abstract
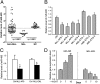
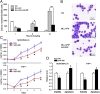
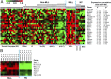
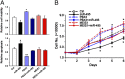
Acute myeloid leukemia (AML) is a heterogeneous group of hematopoietic malignancies with variable response to treatment. AMLs bearing MLL (mixed lineage leukemia) rearrangements are associated with intermediate or poor survival. MicroRNAs (miRNAs), a class of small noncoding RNAs, have been postulated to be important gene expression regulators virtually in all biological processes, including leukemogenesis. Through a large-scale, genome-wide miRNA expression profiling assay of 85 human AML and 15 normal control samples, we show that among 48 miRNAs that are significantly differentially expressed between MLL- and non-MLL-rearranged AML samples, only one (miR-495) is expressed at a lower level in MLL-rearranged AML than in non-MLL-rearranged AML; meanwhile, miR-495 is also significantly down-regulated in MLL-rearranged AML samples compared with normal control samples. Through in vitro colony-forming/replating assays and in vivo bone marrow transplantation studies, we show that forced expression of miR-495 significantly inhibits MLL-fusion-mediated cell transformation in vitro and leukemogenesis in vivo. In human leukemic cells carrying MLL rearrangements, ectopic expression of miR-495 greatly inhibits cell viability and increases cell apoptosis. Furthermore, our studies demonstrate that PBX3 and MEIS1 are two direct target genes of miR-495, and forced expression of either of them can reverse the effects of miR-495 overexpression on inhibiting cell viability and promoting apoptosis of human MLL-rearranged leukemic cells. Thus, our data indicate that miR-495 likely functions as a tumor suppressor in AML with MLL rearrangements by targeting essential leukemia-related genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062. - PubMed
-
- Zhang Y, Rowley JD. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 2006;5(9-10):1282–1297. - PubMed
-
- Rowley JD. Chromosome translocations: Dangerous liaisons revisited. Nat Rev Cancer. 2001;1(3):245–250. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

